Center Points
Dakota Feeder Calf Show Supports NDSU Livestock Research
The 22th annual Dakota Feeder Calf Show is this Saturday, October 17, in Turtle Lake, N.D.
 2019 Show calves at the Turtle Lake Weigh Station
2019 Show calves at the Turtle Lake Weigh Station
Interested consignors deliver 500- to 700-pound calves before 10 a.m. CST on the day of the show. Each producer can consign one or two pens containing three or four calves each. The calves are exhibited and evaluated that afternoon and then shipped to the North Dakota State University Carrington Research Extension Center feedlot to be fed to finished market weight.
NDSU Extension partners with the Dakota Feeder Calf Show to provide producers an opportunity to experience retained ownership of calves beyond the cow-calf segment of cattle production.
There are several ways to collect growth performance carcass data from calves. The best is to feed out an entire calf crop, which takes considerable time, effort and funds. An alternative is to consign a group of calves to a feedout project: risk is decreased and this project provides substantial information about those calves.
During the 2019‐20 feedout, calves gained an average of 714 pounds in 213 days, with a total feeding cost (excluding interest) of 72.8 cents per pound of gain. The average sale weight was 1,344 pounds. The calves were fed with a market weight break‐even point of $106.08 per hundredweight.
In the 2019‐20 feedout, the spread in net return per head between the average of the top and bottom five herds was $99.56. The spread between the top and bottom herd is more noticeable at $124.43 per head. Average daily weight gain in the feedlot was 3.67 pounds for the top‐profiting herd and 3.06 pounds for the bottom herd.
Small differences in production have a huge impact on profit.
CREC staff gather data on the rate of gain, feeding costs and other characteristics during the trial. After the calves are marketed, staff collect carcass weight, meat quality and value data.
Calves should be prevaccinated for BVD, PI3, IBR and BRSV, Mannheimia, Clostridials and histophilus somni. Booster vaccinations will be administered upon delivery to the show.
Producers will be assessed an entry fee of $20 per calf. Dakota Feeder Calf Show officials will present awards to producers at the end of the trial.
For more information or to preregister calves, contact Karl Hoppe at the CREC at 701‐652‐2951, by cell at 701-650-8810, or at karl.hoppe@ndsu.edu, or Darwin Chesrown, Turtle Lake Farmers Union Oil, at 701‐448‐2356.
Karl Hoppe
Extension Livestock Systems Specialist
Karl.Hoppe@ndsu.edu
Soybean harvest season is also time to soil sample for soybean cyst nematode
Soybean harvest currently is a common farm activity. Another activity that farmers are encouraged to do during early October is to soil sample for soybean cyst nematode (SCN). This task can actually be done before or after harvest (or during harvest while you are waiting to fill a grain wagon or truck), and will coincide with the highest SCN egg levels in the soil.
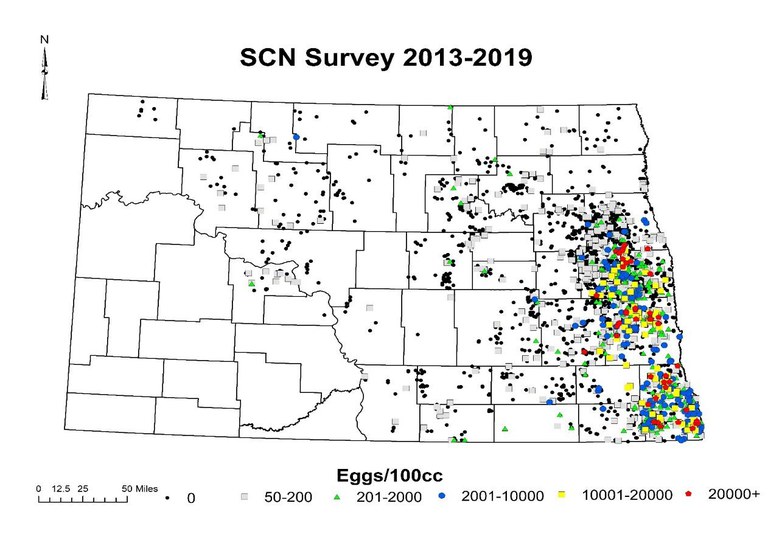
Soil analysis results for SCN in North Dakota from 2013-2019.
NDSU Extension, in cooperation with the North Dakota Soybean Council (who provides funding for soil analysis), has information and bags for soil sampling that can be obtained at county Extension and Research Extension Center offices.
SCN is a parasitic nematode that will complete two to three life cycles each growing season. Each female will produce 100-200 eggs, which are protected within her body wall after she dies (called a ‘cyst’). When we soil sample, we are measuring the amount of eggs in the soil.
When sampling, aim for the roots, sample right next to the plant or harvested row. You only need to go 6-8 inches deep. Take 10-20 small samples, mix, and fill soil bag with the composite sample.
SCN moves with anything that moves soil, loves high pH, and often doesn’t show above ground symptoms. Consequently:
Focus on areas that are likely to introduce soil to your field, such as:
- Field entrances (SCN being moved on equipment with soil)
- Low spots where water pools (SCN moving in water)
- Frequently flooded areas (SCN moving in water)
- Along shelter belts or fence lines (SCN moving with wind-dispersed soil)
Additionally, several areas should be considered suspicious for SCN, and can be sampled.
- High pH spots in the field (SCN loves high pH).
- Areas with unexplained low yields (do the beans look fine but yield poorly in a spot?).
- Areas that turned yellow in August (heavy SCN damage may show up as stunted or yellow beans when it is hot and dry late in the season).
Results from soil analysis indicate eggs per hundred cc of soil (eggs/100cc). Samples with 50-200 eggs indicate very low levels and should be viewed as suspicious (but not necessarily confirmed). Numbers greater than 200 eggs should be viewed as a positive, and, should be managed. SCN can explode very quickly, so a find of 1,000 should be aggressively managed, so it doesn’t become 10 or 50 times higher after the next planting of soybeans or dry beans.
Adapted from information written by Sam Markell, NDSU Extension plant pathologist.
Greg Endres
Extension Cropping Systems Specialist
Gregory.Endres@ndsu.edu
Corn Silage 2020
Frost came early in September 2020 and many producers are wondering about silage quality.
Traditionally, frost signals the beginning of corn-chopping season. After the frost, the plants begin to lose moisture. When the corn plant reaches 63-68 percent moisture, optimal for the ensiling process, harvest begins.
This year, most corn plants were still maturing when an early frost nipped most of North Dakota. When the intent is to harvest corn for silage, producers plant a later-maturing corn variety to take advantage of higher tonnage yields. These plants were wet and seemed to have held their moisture after the frost. But appearances are deceiving; while the corn leaves lost color and dried out, the rest of plant hadn’t quickly lost moisture. Silage piles around the Carrington area were made at 62-67% moisture two weeks after the frost.
Consider that corn leaves are only a small percentage of the weight in a corn plant. Most of the corn plant weight is in the grain, cob, and stalk. While leaves contribute some feed value to the corn silage pile, most of the silage volume is from the rest of the plant.
 Frosted late-season corn didn’t reach maturity
Frosted late-season corn didn’t reach maturity
Silage yields are reportedly lower this year that previous years. This could be due to the frost and the subsequent early harvest. Some fields were already drying down prior to the frost due to a lack of ground moisture. Other fields were only partially damaged by the frost and only the upper part of the plant was damaged.
 Frost damage stopped just above the ear in a field near the Canadian border
Frost damage stopped just above the ear in a field near the Canadian border
Making good silage starts with sufficient moisture. When chopped too wet (greater than 68% moisture) horizontal silage piles may seep moisture. If corn is chopped at more than 80% moisture, improper fermentation will occur and a rancid smell may develop. When chopped too dry (less than 60% moisture), the silage pile will ferment but never reach a stable acidic pH. In 62-68% moisture silage, microbes stop growing when enough acid is produced during fermentation. This stabilizes the silage for future use.
Silage that is put up too dry continues to ferment through the feeding season. It will be warm, develops a brown color, and has a caramel scent. Continued microbial heating causes nutrient loss from the silage pile. While cattle may eagerly consume drier silage, nutrient loss and spoilage is greater than in a properly-preserved 62-68% moisture silage.
Inoculants can help stimulate the fermentation process. These are aggressive microbes that quickly drop silage pH. Covering a silage pile with plastic to reduce moisture loss will reduce spoilage.
 Good fermentation will have lower silage pile temperatures. This picture was taken five days after chopping. The internal temperature is already dropping indicating good fermentation.
Good fermentation will have lower silage pile temperatures. This picture was taken five days after chopping. The internal temperature is already dropping indicating good fermentation.
Karl Hoppe, Ph. D.
Karl.Hoppe@ndsu.edu
Extension Livestock Systems Specialist
How N Stabilizers Impacted Wheat Yields at Carrington in 2020
Nitrogen (N) stabilizers prevent loss of N from N-based fertilizers. Because they are more expensive than plain urea or UAN (28%), their use for crop production should be limited to conditions where the fertilizer application on soil surface will likely result in N loss (e.g. wet soils).
Four field trials were conducted at Carrington to examine the effect on wheat yields of some newly developed slow release N stabilizer formulations (RVix and Pur, which are coded names) that are still undergoing field tests, in comparison with the yields from plain urea and other established N stabilizers like Agrotain, SuperU, and ANVOL.
Urea compared to Agrotain and ANVOL: Fig 1. A and B. Conducted under dryland and irrigated conditions, yields were not significantly impacted by N fertilization. As a result, yields were not different between urea, Agrotain and ANVOL at any of the four N rates (60, 90, 120, and 160 lbs/ac).
Urea compared to Pur (15, 30, 60), ESN and Agrotain: Fig 1. C. Even though yields improved from N application; however, yields were not significantly different between N sources.
Urea compared to RVix, ESN and SuperU: Fig 1. D. Yields were again not different between tested N stabilizer formulations and urea.
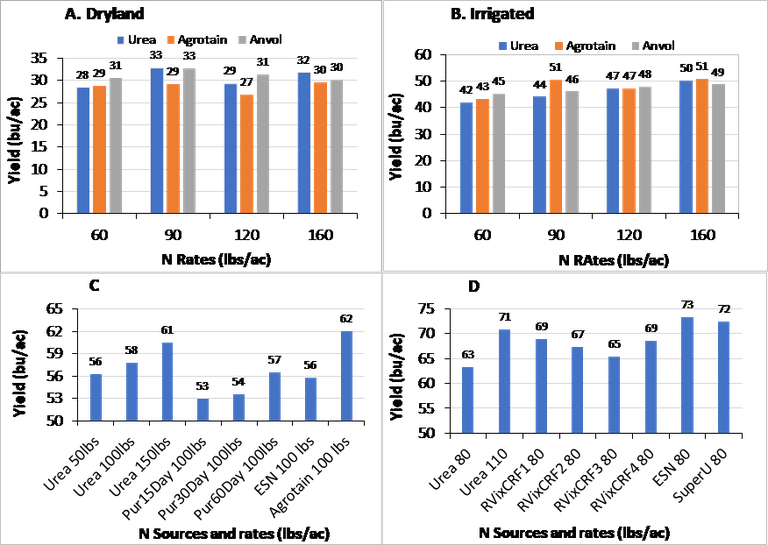 Wheat yield response to urea N compared to N stabilizers (urease inhibitors) under dryland (A) and irrigation (B), and compared to slow release N fertilizers, C and D.
Wheat yield response to urea N compared to N stabilizers (urease inhibitors) under dryland (A) and irrigation (B), and compared to slow release N fertilizers, C and D.
Conclusion
Nitrogen stabilizers did not provide any real yield benefits following their application to spring wheat. Even though a few of these products are proven to be efficient at reducing N losses, any yield gain, or any yield loss prevention that may result from N stabilizer use must important enough to justify their use. Farmers should avoid buying new products without the scientific data to support the results.
Remember to only buy and use N stabilizers for what it is proven to do, to protect N loss, and to be applied when it is most needed.
Previous article on N stabilizers at https://www.ag.ndsu.edu/carringtonrec/center-points/some-things-to-know-if-you-are-planning-to-use-nitrogen-stabilizers
Jasper Teboh, Ph. D.
Jasper.Teboh@ndsu.edu
Soil Scientist
Drying Apples
How is your apple tree doing? Are you getting lots of apples? This year, my favorite ‘Zestar!’ trees at the CREC orchard aren’t producing many. Plenty of ‘Hazen’ apples and trees full of ‘Honeycrisp’ are on the way if the weather holds out past this recent cold spell. All the varieties are ripening later than normal – like last year.

Apple harvest, September 18, 2019
How the weather affects our plants is so mysterious. ‘Zestar!’ blooms first, and has a light crop this year though it’s been a very reliable bearer in all other years. The three middle varieties, ‘Honeycrisp’, ‘Haralred’ and ‘Hazen’ are loaded, while the latest variety, ‘Sweet 16’, is almost bare. The bloom period had nice weather and we had honey bees already. But think back to last fall… 4” of rain in mid-September, 20” of snow October 10 with freezing temps followed by warming weather. Who knows how these events affected the forming buds!
Here is one of our favorite things to do with apples: dehydrate them. I’ve tried drying all our varieties and only ‘Zestar!’ has really good flavor in the end. (I think it’s the exclamation point in the name.) We snack on them all winter and use them in yogurt or oatmeal. I don’t bother dipping the slices into ascorbic acid. The difference in browning of the finished slices is hardly noticeable.
 'Zestar!' apple rings ready to go
'Zestar!' apple rings ready to go
 About 8 hours later they're ready!
About 8 hours later they're ready!
There are plenty of dehydrating resources from Extension programs all over the country. Here is NDSU’s: Food Preservation: Drying Fruits (FN1587, Reviewed Nov. 2017)
P.S. I dried ‘Somerset Seedless’ grapes a couple years ago. I blanched them as suggested and yes, they took about 24 hours to dry! They were tangy and tasty.
Kathy Wiederholt
Fruit Project Manager
Kathy.Wiederholt@ndsu.edu
Hello from David Kramar
Hi, Folks! My name is David Kramar, and I am the new Precision Agriculture Specialist for the Carrington Research Extension Center.

David Kramar, Ph. D., is the Precision Agriculture Specialist at the Carrington Research Extension Center.
To give y’all a bit of information about me:
I completed both my Master’s degree in Geography, and my Ph.D. in Geospatial and Environmental Analysis at Virginia Tech. I have been involved in the Geospatial industry now for over 20 years and have worked across nearly every discipline. During the last year of my undergraduate degree from Appalachian State I worked in local government as the GIS Administrator and Zoning Administrator for Banner Elk, NC. From there I went into the private sector where I held positions as a municipal GIS analyst and Senior Project Manager. After leaving the private sector to go back to school, I began working in non-profit and environmental research. My master’s research was focused on mercury in common loons, and my doctoral research was focused on mercury in bald eagles. In fact, I still climb into bald eagle nests to collect samples every chance I get! In between finishing my doctorate and moving on, I worked as a research associate for the Conservation Management Institute at Virginia Tech.
Since completion of my doctoral degree, I have served as a Visiting Assistant Professor at Southern Oregon University, and more recently as an Assistant/Associate Professor at Minnesota State University Moorhead. Over this time period, my research shifted focus to developing methods and techniques for utilizing ultrahigh resolution UAS imagery for understanding rangeland and agricultural ecosystems. This was the rationale and how I ended up at Carrington. Without question, I am a “boots on the ground” and “fieldwork” individual. I look forward to meeting and working with everyone, and further developing our understanding and use of geospatial technologies as they apply to agriculture in North Dakota. Precision Agriculture is about data, maps, and spatial processes – three things that are inherently intertwined.
“Maps are like campfires –
everyone gathers around them, because they allow people to understand complex issues at a glance,
and find agreement about how to best help the land”.
- Sonoma Ecology Center
David Kramar, Ph.D.
Precision Agriculture Specialist
David.Kramar@ndsu.edu
Summer Roundup
As 2020 started, most of us had great optimism for the new year, but that quickly changed and we have all pivoted and altered our course. It’s amazing to think that this year is now two-thirds over. One advantage during these times of social distancing is more time focused on wrapping up projects and clearing things off the ‘to do’ list. One thing is certain, social distancing gave me plenty of time to accomplish these tasks.
One such project was publication on the pen cleaning study from the winter of 2018/2019. While our study was not able to show improvements in steer average daily gains, we were able to demonstrate that greater extent of pen cleaning increased marbling score and subsequently improved carcass quality grades. For those of you interested in more detail about this study please feel free to reach out and I can share a link to the publication, or you can access the abstract at https://doi.org/10.1016/j.livsci.2020.104204.

Steers at bunk on corn silage, corn, and distillers grain based feedlot rations
Meanwhile, Carrington REC Research Specialist Wayde Rodehorst and NDSU Animal Science graduate student Becca Moore have been working on research, too. They recently wrapped up three projects, including a pair of studies on the use of soybean hulls in beef cow/calf and feedlot rations, and a metabolism project looking at roughage inclusion rates in wheat-based feedlot rations.
Of course, the livestock crew has been l, baling hay and straw, fixing corrals and fences, and making sure that the day-to-day operations at the CREC livestock unit continue without a hitch as fall feeding, weaning, and eventually winter arrive.
Moving forward, we will start a number of new research projects including evaluation of roughage sources in feedlot rations. We are collaborating on research using new and emerging feed ingredients in beef cattle rations. We will also be conducting a second receiving and finishing project on steers from dams managed under different trace mineral and vitamin regimens in early gestation and during lactation. This project will allow us to gain greater understanding of how previous trace mineral programs impact beef calves’ ability to manage stress associated with weaning and the transition into feedlot settings.
Anyone interested in more information about our research projects should reach out for a visit or tour.
Bryan Neville
Bryan.Neville@ndsu.edu
Animal Scientist
Virtual Row Crop Tour Starting on August 27
The Carrington Research Extension Center is holding its annual row crop tour virtually this summer because of the COVID-19 pandemic. This format will meet the objectives of our tour by providing updates on the center’s corn, dry bean, soybean and sunflower research and recommendations for farmers and crop advisers. Ag audiences will be able to watch the videos at a time convenient for their schedules.
Prerecorded videos are targeted to be available August 27 and can be found at https://www.ag.ndsu.edu/CarringtonREC/videos
Topics and NDSU speakers for the program are:
- Incorporating cover crops into corn - Mike Ostlie, CREC research agronomist
- Impact of nitrogen fertilizer rates on corn and dollar return in low and high yield environments - Jasper Teboh, CREC soil scientist
- Dry bean breeding program and variety review – Juan Osorno, dry bean breeder
- Black, navy and pinto bean plant populations and row spacings - Endres
- Pollinator potential impact on dry bean - Savannah Adams, graduate student
- Optimizing fungicide spray droplet size for improved management of white mold in dry bean - Michael Wunsch, CREC plant pathologist
- SCN considerations for dry bean and soybean - Sam Markell, Extension plant pathologist
- Considerations for soybean variety selection - Hans Kandel, Extension agronomist
- Prospects for managing Sclerotinia head rot in sunflower - Wunsch
- Susceptibility of sunflower to Sclerotinia head rot relative to growth stage - Wunsch
 Pinto bean trial testing three row spacings and three plant populations
Pinto bean trial testing three row spacings and three plant populations

Sunflower in bloom – susceptible stage for sclerotinia head rot
Greg Endres
Gregory.Endres@ndsu.edu
Extension Cropping Systems Specialist
What Happens if EAB Comes to Your Town?
For years now, we have been warned that emerald ash borer (EAB) is a threat to North Dakota ash trees. Since its 2002 discovery in Detroit, EAB has continued to spread across North America and toward us, killing millions of ash trees. EAB is established and is destroying ash trees in neighboring Minnesota, South Dakota and Winnipeg, Manitoba (see the map at www.emeraldashborer.info). Although EAB has NOT been detected in North Dakota (as of the time of this writing), its arrival in ND is inevitable. It will spread in a sporadic fashion – it will not engulf the state all at once. But all ash trees are at risk and the costs in North Dakota will be devastating.
 Adult Emerald Ash Borer. The immature or larval stage of this pest tunnels into the trunk and branches of ash trees, cutting off the flow of water, nutrients and sugars under the bark and killing the tree in a few years.” Photo credit: David Cappaert, Bugwood.org
Adult Emerald Ash Borer. The immature or larval stage of this pest tunnels into the trunk and branches of ash trees, cutting off the flow of water, nutrients and sugars under the bark and killing the tree in a few years.” Photo credit: David Cappaert, Bugwood.org
What Can You do to Prepare?
Be aware of your tree inventory. The ND Forest Service has completed street tree inventories in more than a hundred communities, visible for viewing on the ND TIP Tool website, http://ndcitytrees.org. Click on the ‘Explore Cities’ button to find your town. Public trees will show up as dots, color-coded to show different types of trees. In nearly all ND communities, ash trees (this includes all cultivars of green ash and black ash) dominate streets and the landscape. ON PRIVATE PROPERTY: Note the ash trees in your yard and in windbreaks and shelterbelts. Are they important for shade? Wind protection? Wildlife habitat? These valuable trees may someday be gone, thanks to EAB.
 Many streets in North Dakota communities are lined with green ash trees like these in Milnor. Photo credit: Mary O’Neill, North Dakota Forest Service
Many streets in North Dakota communities are lined with green ash trees like these in Milnor. Photo credit: Mary O’Neill, North Dakota Forest Service
Remove trees that are in decline. Communities can view their inventory to identify unhealthy trees. Remove them ASAP, especially ash trees, to make your streets safer, more beautiful, and to open planting spaces for a variety of new trees. Many ash trees are in decline due to physical stresses and native pest problems that attack stressed trees.
 Dead and dying green ash in Fort Snelling, Minnesota. Streets In North Dakota may someday look the same.” Photo credit: Ralph Sievert, Minneapolis Park and Recreation Board Forestry Department.
Dead and dying green ash in Fort Snelling, Minnesota. Streets In North Dakota may someday look the same.” Photo credit: Ralph Sievert, Minneapolis Park and Recreation Board Forestry Department.
Plant a variety of new trees. Diversity of trees will limit the impact of any pest or disease. Choose the right tree for the site – for open spaces, plant larger statured trees like lindens, hackberry, Kentucky coffeetree and Dutch elm disease resistant-elms. Medium-size trees include Ohio buckeye and honeylocust. For narrow boulevards and areas near overhead wires, choose smaller-size trees like flowering crab, Amur maple and Japanese tree lilac.
Don’t Move Firewood. The most common way for invasive pests to spread is through the transport of firewood. Burn harvested wood locally, or buy it where you plan to burn it. Don’t move firewood!
Contact ND Forest Service for assistance. For communities, NDFS Community Forestry cost-share grants are available for tree removals and for planting trees on public property. Rural landowners can receive technical assistance to evaluate existing plantings and develop a management plan.
For more information on community tree inventories, tree selection, or community forestry grants, contact NDFS Community Forestry staff:
Gerri Makay (Carrington) Gerri.Makay@ndsu.edu
Mary O’Neill (Lisbon) Mary.Oneill@ndsu.edu
Beth Hill (Bismarck) Beth.Hill@ndsu.edu
For questions about windbreak assessments, contact NDFS Forest Stewardship Manager
Liz Smith Liz.Smith@ndsu.edu
Gerri Makay
Gerri.Makay@ndsu.edu
Community Forestry Manager
Surprise!
For this week’s Center Points post, Mary was going to remind you that there is still time to register for the online composting workshop and live webinar with producers that happens on August 11. You can register here: www.tinyurl.com/manurecompostingworkshop.
Mary was also going to remind you that it’s time to start thinking about calibrating your manure spreaders and share this helpful publication: Manure Spreader Calibration for Nutrient Management Planning. There’s also a useful video from her colleagues at UMN Extension on solid spreader calibration.
Instead, she asked us to introduce Lucy Keena, who arrived a little ahead of schedule. Lucy, Mary and Nick (dad) will be hanging out in the NICU for a few weeks while she continues to grow. She’s a healthy “little spitfire”, the nurses say! Mary will be on leave for a couple months so until she’s back please send Center Points comments, questions or suggestions to Linda.Schuster@ndsu.edu.
Lucy Keena is the newest addition to the NDSU Carrington Research Extension Center family.
Linda Schuster
Linda.Schuster@ndsu.edu
Administrative Secretary


