Water Softening (Ion Exchange) (WQ1031, Revised Aug. 2017)
Availability: Web only

Standard water softeners found in home supply and hardware stores will remove nearly all the calcium and magnesium from the raw water during the softening process. Softeners also will remove up to 10 parts per million (ppm) of iron and manganese. Water supplies with high levels of iron and manganese (greater than 10 ppm) may need pretreatment to prolong the lifespan of a water softener.
What Makes Water “Hard”?
So-called “hard” water is the result of ground water passing through and dissolving rocks and minerals, which then release calcium and magnesium ions. These dissolved ions give hard water its characteristics.
How is Hardness Measured?
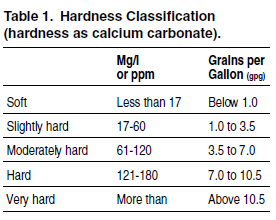
Water hardness is expressed in grains of hardness per gallon (gpg) of water or as parts per million (ppm) or milligrams per liter (mg/l). One gpg is equal to 17 ppm (mg/l). Table 1 shows how hardness is classified. According to NSF International, a public health and safety organization, consumers believe they have a problem when hardness surpasses the 6 to 7 grains-per-gallon level.
Problems Caused by Hard Water
Hard water interferes with all types of cleaning tasks. Cleaning problems arise when the cleaning agents do not fully remove dirt and grime. Through time, clothes washed in hard water may look dingy and feel harsh and scratchy. White clothing continually washed in hard water gradually will become dingy.
Dishes and glassware washed in dishwashers using hard water may be spotted when dry. Hard water causes films on glass shower doors, walls and bathtubs. Hair washed in hard water may feel sticky and look dull. After showering in hard water, skin will feel rough and scratchy. This feeling is especially noticeable in the winter when the air’s relative humidity is low.
Scum occurs when soap combines with dissolved calcium and magnesium. Soap scum is difficult to remove from sinks, showers, bathtubs and other appliances.
Hard water use may affect the performance of household appliances. When heated, calcium carbonate and magnesium carbonate settle out of suspension (precipitate) from the water and form a mineral deposit at the bottom of the hot water heater. A large scale buildup slows the heating process and requires more energy to heat water. Water heaters with large accumulations of mineral buildup will have a shorter life span. Scale deposits also accumulate to plug plumbing fixtures and build up in other appliances such as coffee pots and dishwashers, thus affecting their performance.
The Ion Exchange Process
Calcium (Ca2+) and magnesium (Mg2+) ions that cause water hardness can be removed fairly easily by using an ion exchange procedure. Standard water softeners are cation exchange devices. Cations refer to positively charged ions dissolved in the water. Cation exchange involves the replacement of the hardness ions with a nonhardness ion.
Water softeners usually use sodium (Na+) as the exchange ion. Sodium ions are supplied from dissolved sodium chloride salt, also called brine. In the ion exchange process, sodium ions are used to coat an exchange medium in the softener. The exchange medium can be natural “zeolites” or synthetic resin beads that resemble wet sand.
As hard water passes through a softener, the calcium and magnesium trade places with sodium ions (Figure 1). Sodium ions are held loosely and are replaced easily by calcium and magnesium ions. During this process, “free” sodium ions are released into the water.
Softening Process
After softening a large quantity of hard water, the exchange medium becomes coated with calcium and magnesium ions. When this occurs, the exchange medium must be recharged or regenerated (Figure 1).
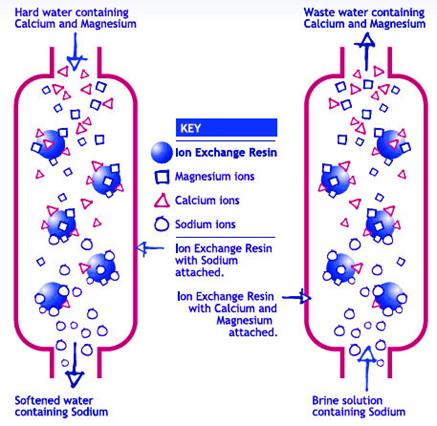
To recharge the softener with sodium ions, a softener is backflushed with a salt brine solution. During a backflush, the brine solution replaces the calcium and magnesium ions on the exchange medium with sodium ions from the salt solution.
Recharging Process
The time between recharging cycles depends on the hardness of the water, the amount of water used, the size of the unit and the capacity of the exchange media to remove hardness.
Have Your Water Tested
Before buying any water treatment equipment, you should know what impurities are in the water supply. A certified laboratory can determine the types and amounts of impurities in your water. A list of certified labs is available in the NDSU publication “Drinking Water Quality: Testing and Interpreting Your Results” (WQ1341). The results of the water test will help determine if softening is needed. The water testing may reveal if other water treatment is required.
If you obtain water from a private water supply, water testing is your responsibility. Water testing should be done on a regular basis. If you suspect a problem, test more often.
Community water supplies are monitored and treated to protect users from health-threatening water impurities. Ask your supplier for a copy of the latest water test results.
Hard water is considered a nuisance water problem. Hardness removal is not a necessity to protect your health, but water softening is popular because most people prefer softened water for bathing, cleaning and washing.
Health Risks Associated With Softened Water
During the softening process, sodium is released from the exchange media into the output water. For every grain of hardness removed from water, 8 mg/1 (ppm) of sodium is added. People on restricted sodium intake diets should account for increased levels of sodium in softened water. Your family physician should be consulted.
Sodium intake from softened water can be avoided by leaving one kitchen tap unsoftened for drinking and cooking. Substituting potassium chloride for sodium chloride may be appropriate if health or environmental reasons necessitate restricting sodium.
Potassium chloride is more expensive and adheres more strongly to the resin, reducing the exchange efficiency when compared with sodium chloride. About 10 percent more potassium chloride salt is used during the backflush operation.
Types of Water-softening Equipment Available
The primary difference among water softener models is in the way they schedule the backflush cycles. This is important because recharging the beads with salt too early wastes salt and water. Recharging too late causes performance to fall off.
Water softeners are classified into four different categories:
• Semiautomatic: The operator initiates only the recharging cycle. The operator pushes a button when the softener needs recharging, and the unit will control and complete the recharging process.
• Automatic: Automatic softeners have a timer that automatically initiates the recharging cycle and every step in the process. The operator sets the timer and adds salt as needed. This is the most common type of softener used.
• Demand-initiated regeneration (DIR): All operations are initiated and performed automatically in response to the water use demand for softened water. DIR systems generally have two softening tanks and a brine tank. While one tank is softening, the other tank is recharging.
• Off-site regeneration (generally rental units): A used softening tank is replaced with a recharged tank. Spent softening tanks then are recharged at a central location.
Operation and Maintenance
Maintenance of water softeners largely is confined to restocking the salt supply for the brine solution. With semiautomatic models, the owner also will have to start the recharging cycle. Salt can be purchased in the form of pellets, granules or blocks.
The brine tank may require periodic cleaning. The frequency of cleaning depends on the amount and purity of the salt used in the softening process. The brine valve and float assembly also should be checked and cleaned as often as needed.
The presence of excess iron or hydrogen sulfide can inhibit the effectiveness of a water-softening unit. Installation of the iron-removal equipment may be required (see the NDSU publication “Iron and Manganese” (AE1030) . Water test results will help make that determination.
More frequent backflushing (reversing the normal flow of water through the treatment unit) may be required to remove iron buildup.
Cost of Water Softeners and Supplies
Retail prices for home water softeners may range from approximately $400 to $2,700, depending on the size and type of softener. Softeners are rated by the total number of grains the unit can remove before being recharged. The cost of salt is approximately $5 to $8 per 40-pound bag of sodium chloride (NaCl), depending on the form purchased, and up to $25 per 40-pound bag for potassium chloride (KCl).
Advantages of Water Softeners
Softeners offer cleaner, softer feeling clothes; longer life of appliances, including washing machine, dishwasher and water heater; less use of household cleaning products, such as detergents, as well as personal cleanliness products such as shampoo; and reduction of water spotting.
Disadvantages of Water Softeners
Softened water is not recommended for watering house plants, lawns and gardens due to its sodium content. Water used in recharging a water softener may overload or reduce the effectiveness of small septic or sewer systems. Sodium intake may pose health risks. Softened water is not recommended for steam irons or evaporative coolers. The best choice for such appliances is distilled water or water from a reverse osmosis unit.
Alternatives to Ion Exchange Units
Hard water problems can be reduced by using detergents that include water-softening chemicals in their formulation. Some types of chemicals can be added to hard water to reduce the negative effect of calcium and magnesium. Chemical treatment for household water softening is recommended for low levels of hardness.
Two types of chemicals used to soften water for home laundry are Sal Soda and Calgon.
Sal Soda combines with calcium and magnesium to form solid particles. These particles settle out with particles of dirt during washing. Use of precipitating additives such as Sal Soda may not fully clean your laundry because solid particles may cling to fabrics.
Calgon softens water by combining with calcium and magnesium to form compounds that stay in solution. The use of nonprecipitating additives such as Calgon has a negative environmental effect because they have high phosphate content that promotes algae growth
in local waters.
Magnetic Conditioning
Permanent magnetic water conditioning devices have been marketed based on a variety of claims regarding their effect on water hardness and related scale formation. The Water Quality Association, a not-for-profit trade association for the water treatment industry, has not been able to determine standards for these products and report inconsistent performance to date. Research has shown no change occurs in the physical and chemical properties or the calcium ion concentration of water treated with the devices (The Water Quality Association Magnetics Task Force, 2001).
Items to Consider When Purchasing an Ion Exchange Water Softener
• Test your water to determine the hardness and other impurities that may need to be removed.
• Determine how much softened water your household needs per day, per year.
• What type and size of softener will fit your situation?
• How easy is the softener to clean and/or repair?
• Will the dealer provide service?
• What type of convenience level should a softener offer (manual or automatic operation)?
• Will pretreatment be needed for iron and manganese?
• Will sodium intake be a health problem?
• Will sodium salts overload your septic or sewer system?
• Investigate equipment before purchasing or renting. Don’t rush a purchase.
• The purchase price does not directly indicate a softener’s performance. A moderately priced unit might work as well as an expensive unit.
• When buying or renting, are the installation costs included in the price?
• Don’t buy more equipment than you need. Other removal systems might be better suited for the removal of certain impurities.
• Choose a reputable dealer. Get guarantees in writing and read them thoroughly.
• Beware of manufacturers’ advertising that is too good to be true.
• Equipment should carry UL and NSF or WQA approval.
Further Information
For further information, contact your local county Extension office or state health department. Additional information can be found in these publications:
• WQ1029 Filtration: Sediment, Activated Carbon and Mixed Media
• WQ1030 Iron and Manganese Removal
• WQ1341 Drinking Water Quality: Testing and Interpreting Your Results
• WQ1352 What’s Wrong With My Water? Choose the Right Test
References
Gruber, C.E., and D.D. Carda. 1981. Measurable parameters in water conditioning equipment as determined in laboratory simulations at Rapid City, S.D. Final report issued to the Water Quality Association. South Dakota School of Mines and Technology.
WQA Magnetics Task Force Report. (2001). The Water Quality Association Magnetics Task Force. Water Quality Association (Order No. R20, FGI-3M) (Pamphlet). Lisle, Ill.
Private Water Systems Handbook. (5th Ed.) MWPS-14. Midwest Plan Service. ISBN 0-89373-105-6. April 2009.
The NDSU Extension Service does not endorse commercial products or companies even though reference may be made to trade names, trademarks or service names.
This publication was authored by Roxanne Johnson, former Water Quality Associate, and Tom Scherer

