Soil Testing Unproductive Areas (SF1809, June 2016)
Availability: Web only
Is This Soil Saline, Sodic or Saline-sodic?
Most producers have areas in their fields that are not productive (Figure 1). High soluble salts (soil salinity) or excessive sodium (soil sodicity) levels are usually the cause.

Figure 1. A salt-affected unproductive area next to productive land with canola in Walsh County, N.D.
(Naeem Kalwar, NDSU)
A soil can be mapped as very fertile; however, it may not be productive due to high salinity and/or sodicity. Soils with high salinity/sodicity usually have adequate levels of essential plant nutrients because the nutrients are not being removed at harvest due to low grain/fodder production, compared with more productive areas of the field.
The problem is not the low nutrient levels but an excess of soluble salts that restricts crop growth and development and/or excessive sodium that deteriorates soil structure.
The ongoing wet weather cycle experienced in this region since the early 1990s has led to high groundwater levels and, in many cases, has resulted in saturated soils. When the rain is heavy, soils with high groundwater levels are not able to absorb a lot of water and get saturated quickly, compared with soils with low groundwater levels.
Saturated soils do not allow for increased infiltration and, therefore, surface runoff will occur. Also, the soil environment is not conducive for plants and microorganisms because they require oxygen to respire, and the oxygen is limited due to the saturated conditions.
Because the groundwater in our region naturally contains high levels of soluble salts (CaSO4, MgSO4, Na2SO4), the ions of these salts (Ca2+, Mg2+, Na+, SO42-) wick to the soil surface through capillary water movement. Once the water content becomes less than the solubility of the salts, the ions recrystallize and can be seen as “salts” on the soil surface or within the soil profile.
The increase in soil salinity and sodicity are serious soil health issues that result in poor crop yields and frequently leave affected areas bare of plant growth. Understanding how high salt and high sodium levels affect plants and soils, learning to recognize the visual symptoms, properly sampling the affected areas, knowing what soil tests to ask for and understanding the results are crucial steps to remediate these areas for profitable crop production.
What Do Excessive Salts and Sodium Do to Plants and Soils?
Effect of High Soluble Salt Levels
Soluble salts are a combination of positively and negatively charged ions (for example, table salt; Na+Cl-). High levels of ions (positive and negative) from soluble salts restrict normal water uptake by plant roots, even when soils are visibly wet, resulting in drought-stressed plants.
The underlying process that produces drought symptoms in the presence of normally adequate water is called the “osmotic effect.” Soil water moves from higher osmotic potential to lower osmotic potential. Osmotic potential reflects how freely soil water can move from one point to another.
Water molecules have two positive charges supplied by two hydrogen (H+) atoms and one negative charge provided by one oxygen (O2-) atom. With their negative side, water molecules get attracted to positively charged ions, and with their positive side, they get attracted to the negatively charged ions and soil particles such as clay and humus. High levels of soluble salts pull water molecules strongly toward them, thus resulting in lower osmotic potential.
In soils with low soluble salt levels, plant roots accumulate more salts in their root cell membrane than soil water and are able to pull (absorb) water. However, in soils with high salt levels (saline soils), the pull for water is strong within the soil itself and water doesn’t readily move toward plant roots.
Saline soils having higher levels of calcium (Ca2+)-based salts, compared with soils high for magnesium (Mg2+)- and sodium (Na+)-based salts, will have good structure (high “tilth” qualities), unlike sodic soils, resulting in free water movement through the soil profile. That happens as calcium (Ca2+) ions encourage aggregation of soil particles called flocculation (clumping together), resulting in well-defined pores through which water can move.
Effect of High Sodium Levels
In contrast to saline soils, pure sodic soils have extremely poor physical conditions (poor soil structure) with dense soil layers, resulting in very slow permeability of water and air through the soil profile.
The poor structure of sodic soils is due to three reasons:
- High sodium levels in combination with low salt levels can promote “soil dispersion,” which is the opposite of flocculation. Ions such as sodium (Na+), and in some cases magnesium (Mg2+), cause the breakdown of soil aggregates (soil dispersion), resulting in poor soil structure (low “tilth” qualities). Forces that hold clay particles together with soil aggregates are weakened greatly when excessive sodium (Na+) ions are attached to the clay particles and when wet clay particles break away easily from soil aggregates.
- As excessive sodium (Na+) levels increase, the tendency of soil aggregates is to disperse, and the released clay and silt particles then clog soil pores when washed down the soil profile.
- When highly saturated with sodium (Na+) ions, the degree of the swelling of expanding-type clays (smectite) such as we have in the region increases. As these soils swell (expand), the larger pores responsible for water drainage are constricted (Brady, C.B., and Weil, R.R. 2008. Pages 420 and 422, Chapter 10, “The Nature and Properties of Soils,” 14th edition, revised).
Due to poor soil structure, when wet, sodic soils will be gummy and may seem like they have “no bottom” to them, and when dry, they can be very hard.
Is My Soil Saline or Sodic?
Soils with very high soluble salt levels may show a white salt crust at the soil surface. When these soils are wet, the white crust does not show. Soils with a sodium problem usually do not have a white crust at the surface unless the soils also are affected by high soluble salts (saline-sodic soils).
Soil sodicity doesn’t necessarily show clear symptoms at the soil surface, making it more difficult to diagnose casually (Figure 2). The best way to know if a field has sodicity issues is to take a soil sample and have it analyzed by a soil laboratory.

Figure 2. This field near Langdon, N.D., has a saline-sodic area with no clear visual symptom of sodicity at the soil surface.
(Naeem Kalwar, NDSU)
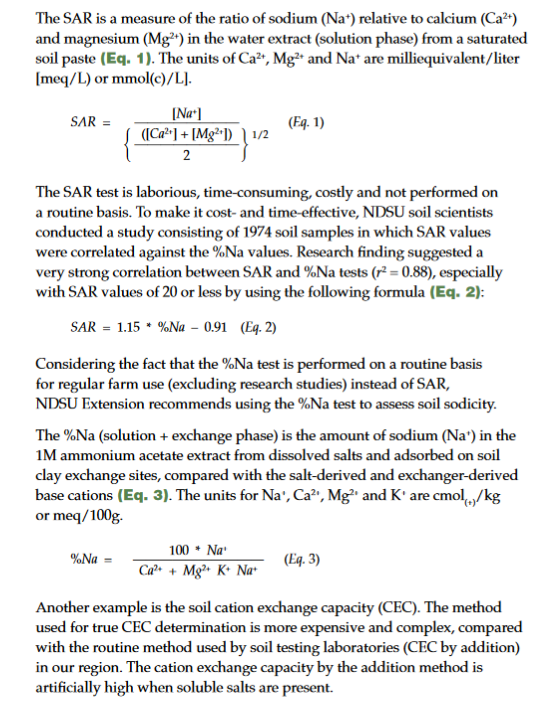
Historically, ESP (exchangeable sodium percentage) or SAR (sodium adsorption ratio) tests have been used to analyze soils for sodicity, especially for research purposes. The ESP (exchanger phase) test, which is the amount of actual sodium (Na+), calcium (Ca2+), potassium (K+) and magnesium (Mg2+) adsorbed on the soil clay exchange sites, has been replaced by SAR in most of the regional soil laboratories through time. As a result, SAR values can be substituted for ESP (Oster et al., 1999).
However, because most soils laboratories in the northern Great Plains analyze samples for percentage of sodium (%Na), NDSU soil scientists recommend using %Na as an acceptable test to measure soil sodicity at the farm level (DeSutter et al., 2015). To determine soil salinity, samples are analyzed for EC (electrical conductivity).
How to Sample Problem Areas
Divide the Areas Into Zones
Before sampling, separate a field with problematic areas into zones based on visual observations and history of plant stands or crop yields (Figure 3). Some areas may support marginal stands, whereas some may be barren. They should be sampled separately based on their unique characteristics.
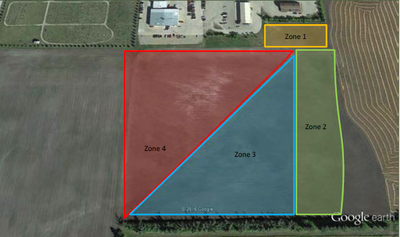
Figure 3. An unproductive area with varying levels of salinity and sodicity divided into four zones based on visual observations, differences in elevation and cropping history at the Langdon Research Extension Center.
Separate samples should be taken from areas that vary in elevation because the thickness of the top soil and levels of salts and sodium will vary from high to low areas. Mixing the samples from areas that are not similar will confound the results and only will provide an average for salt and sodium levels, which will not reflect the true characteristics of each area.
Sampling in zones will result in soil test results specific to each zone, which will contribute to the strategy needed to address the reclamation of the soils within each zone.
Sampling Method
Each zone sample should consist of eight to 10 soil cores to adequately represent the zone (Figure 4). All cores representing a zone should be obtained from the same depths in a soil profile. For example, if we are taking samples to a 3-foot depth in each zone, all eight to 10 cores should be from the 3-foot depth.

Figure 4. A soil core is being taken from a saline-sodic zone with a hand-held auger.
(Naeem Kalwar, NDSU)
For saline and sodic soils, regardless of the depth of sampling, the cores should be separated by no more than 12-inch increments. If taking a 3-foot sample and separating each by 1 foot, use three buckets: the first for mixing the first foot depth, the second for the second foot depth and the third for the third foot depth. Each zone then will consist of three samples: a subsample from the eight to 10 cores at the first foot depth, the second from the second foot depth and the third for the third foot depth.
Sampling Depth
Salts and sodium levels vary with soil depth. Sampling by depth within zones will help determine whether the salt/sodium issue is a surface soil problem or a problem that goes deeper in the soil (Figure 5). Considering the rooting depth of most crops, areas with high salt and sodium levels should be sampled at least 3 feet deep in 12-inch increments. Sampling 4 feet deep in 12-inch increments will be even better (Figure 6).

Figure 5. Soil depth is being separated in 12-inch increments.
(Naeem Kalwar, NDSU)

Figure 6. A 4-feet-deep sample is being separated in 12-inch increments.
(Naeem Kalwar, NDSU)
For tiling, the sampling depth should exceed the deepest depth of the tiles (generally not more than 4 feet) in 12-inch increments. For detailed information, refer to the NDSU Extension publication SF1617, “Evaluation of Soils for Suitability for Tile Drainage Performance.”
What to Test For
Some soil properties, such as texture, do not change with time. However, other properties such as soil salinity and sodicity can change with time due to changes in climate, weather, rising groundwater levels, crop choices and tillage practices. The basic test needed to determine the levels of soluble salts (soil salinity) is EC. For soil Na+ levels (soil sodicity), researchers use the SAR test. Because the SAR test is time-consuming and expensive, at the farm level, %Na is an acceptable test to assess soil sodium (Na+) levels (DeSutter et al., 2015).
Also, many soil laboratories outside of our region do not normally analyze soil samples for these tests, so the best option is to have the samples analyzed for EC (for salinity) and %Na (for sodicity) using regionally based laboratories that understand these soil test procedures and regularly use them.
Other tests that help in developing a remediation strategy are soil pH, chloride (Cl-), sulfate (SO42-), carbonates (CO32-) and bicarbonates (HCO3-). Analyzing the samples for pH is also very beneficial because it is a very good indicator of the availability of plant nutrients and soil chemical properties.
Because most of the soils in North Dakota are shrinking and swelling type of clays, if soil results for %Na are more than 5 and the EC is less than 2 millimhos per centimeter (mmhos/cm), movement of soil water may be restricted due to swelling, dispersion or both.
To remedy soil sodicity, the application of soil amendments will be needed. The amendments will supply calcium (Ca2+), for example gypsum (CaSO4), that supplies Ca2+ directly, or elemental sulfur (S°) that first gets oxidized to sulfate (SO42-) and then coverts into sulfuric acid (H2SO4) that dissolves Ca2+ already present in the soil, for example as lime (CaCO3).
To calculate the rates of soil amendments, soil CEC (cation exchange capacity) test will be required. Soil CEC values can be obtained from the Natural Resources Conservation Service’s Web Soil Survey or by analyzing the soil samples for the CEC test.
Methods of Analysis
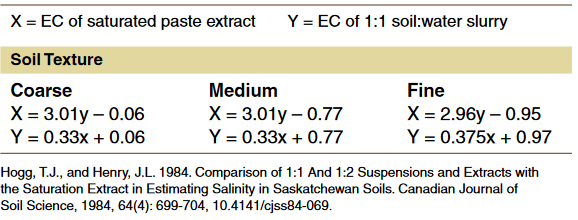
Different labs use different methods to analyze soil samples to assess soluble salt and sodium levels. For salts, for example, the EC values measured with the 1-to-1 by weight soil-to-water method will result in lower values than the saturated paste extract method will provide.
Depending upon soil texture, generally EC values determined through the saturated paste extract method are double or more than the values obtained through the 1-to-1 method. However, the 1-to-1 method is much easier and less expensive to use, and it is the method most used by soil laboratories to evaluate soil salinity levels.
If soil textural values are known, then the formulas in Table 1 can be used as a rough estimate to convert EC values determined through one method to the other. However, considerable variability exits around the conversion values.
For sodium, researchers use the SAR test to assess soil sodicity levels.
Table 1. General conversion from 1-to-1 soil-to-water slurry method used by many commercial labs to the saturated paste extract method used in research applications.
Interpreting Soil Analysis Results
Soil Salinity
For classification purposes, a soil is considered as saline if the saturated paste extract electrical conductivity (EC) equals or exceeds 4 deciSiemens per meter (dS/m). However, in terms of crop production, even an EC of less than 4 dS/m can result in considerable yield loss, for example in the case of soybeans.
Soil EC is a measure of the concentration of ions from water-soluble salts in soils, and the test results are indicative of soil salinity. EC is the ability of a material to conduct an electrical current and it commonly is expressed as dS/m or millimhos/centimeter (mmhos/cm). One dS/m = 1 mmhos/cm.
Soil EC is inversely proportional to the electrical resistance in soil solution. EC is measured by passing an electrical current through the soil solution. Water-soluble salts in the solution enhance the transfer of electric current (electric conductance).
Note: An ohm is a unit of resistance and an mho is a unit of conductance. “Siemens” was adopted as a scientific representation of mho in an 1881
conference in England to honor a prominent scientist of the period who studied electrical conductance. Mho usually is used by lay soil scientists today, although dS usually is used in peer-reviewed scientific journals instead of mho.
Soil Sodicity
Soil SAR and %Na tests measure the levels of Na+ ions in soil water or at soil particle exchange sites and are indicative of soil sodicity.
Because most of the soils throughout North Dakota are shrinking and expanding type of clays, based on NDSU research, soils may start dispersing at a %Na of 5 or more, especially when EC is less than 2 mmhos/cm. Soil dispersion will result in slow soil water infiltration due to the deterioration of soil structure. Analyzing soils for sodium levels before installing tile drainage systems also is very important because dispersion could seal soil layers above or around tiles.
Note: Alternatively, %Na can be substituted for SAR (DeSutter et al., 2015) and SAR can be substituted for ESP in the range of 0 < SAR < 50 (Oster et al., 1999).
For detailed information, refer to the NDSU Extension publication SF1617, “Evaluation of Soils for Suitability for Tile Drainage Performance.”
Management of Soil Salinity and Sodicity
Areas that do not support crop growth still may allow salt/sodium-tolerant weeds, such as kochia and foxtail barley, to grow. These weeds can be allowed to grow and should be mowed before flowering, or they should be hayed or lightly tilled to prevent them from going to seed.
Soil salinity and sodicity require the lowering of groundwater levels. Once groundwater levels are lowered due to natural or artificially installed drainage systems (tile drainage), excessive salts can be leached out of the saline soils with good soil structure and rainfall or snowmelt water. Sodic soils will require an extra step of applying soil amendments that supply calcium (Ca2+) to displace sodium (Na+) from the soil exchange sites and help leach it below the rooting zone.
For detailed information on the management of soil salinity and sodicity, we recommend these resources:
• ”Managing Saline Soils in North Dakota” by D.W. Franzen, 2012. (NDSU Extension publication SF1087)
• “Saline and Sodic Soils” by Naeem Kalwar, 2012.
• Extension soil and fertilizer publications.
• NDSU Soil Health webpage:
Where to Send Samples
Producers can send their samples to a commercial soil testing lab or the NDSU Soil Testing Laboratory for analysis. The mailing address and phone number of the NDSU Soil Testing Laboratory is:
1360 Bolley Drive, Fargo, ND 58102
Phone: 701-231-8942
Email: ndsu.stl@ndsu.edu
For more information regarding NDSU Soil Testing Laboratory
References
Brady, C.B., and Weil, R.R. 2008. The Nature and Properties of Soils, 14th edition (revised), Chapter 10, p-420 and 422.
DeSutter, T., Franzen, D.W., He, Y., Wick, A., Lee, J., Deutsch, B., and Clay, D. 2015. Relating Sodium Percentage to Sodium Adsorption Ratio and Its Utility in the Northern Great Plains. Soil Sci. Soc. Am. J. doi:10.2136/sssaj2015.01.0010n.
Franzen, D.W. 2012. Managing saline soils in North Dakota. NDSU Extension publication 1087. Fargo, N.D.
Hogg, T.J., and Henry, J.L. 1984. Comparison of 1:1 and 1:2 Suspensions and Extracts with the Saturation Extract in Estimating Salinity in Saskatchewan Soils. Can. J. Soil Sci. 64: 699-704.
Oster, J.D., I. Shainberg and I.P. Abrol. 1999. Reclamation of salt-affected soils. Agricultural drainage. Agron. Monogr. 38. ASA, CSSA, SSSA, Madison, Wis.
Ray E.L., and David, A.W. 1992. Management of Saline and Sodic Soils. Kansas State University Agricultural Experiment Station and Cooperative Extension Service MF-1022.
Seelig, B.D. 2000. Salinity and Sodicity in North Dakota Soils. NDSU Extension publication 57.
Soil Survey Staff. 1999. Soil taxonomy: A basic system of soil classification for making and interpreting soil surveys. USDA NRCS Agric. Handbook 436. 2nd ed. U.S. Gov. Print. Office, Washington, D.C. (verified May 22, 2015).
U.S. Salinity Laboratory Staff. 1954. L.A. Richards, editor, Diagnosis and improvement of saline and alkali soils. USDA Agric. Handbook 60. U.S. Gov. Print. Office, Washington, D.C.

