Soybean Production Field Guide for North Dakota (A1172, Revised Apr. 2019)

Introduction
Hans Kandel and Greg Endres, Extension Agronomists
Changing weather conditions with varied rainfall amounts and stored soil water require soybean [Glycine max (L.) Merr.] growers to make careful decisions regarding tillage system, fertility management, variety selection, seedbed preparation, weed control strategies, crop rotations, water management and pest management practices.
This field guide has been developed to help you make timely management decisions. However, detailed and extensive information on any one area is not provided because of limited space. Complete discussions of soil fertility; weed, disease and insect control; variety performance; harvesting; and storage are available in other Extension publications as listed in the back pages.
The pesticide use suggestions in this guide are based on federal label clearances and on some state labels in North Dakota. Also, suggestions are based on research information collected in North Dakota State University experiments or trials in other states. All pesticides listed had a federal or state label at the time of publication of this guide. Check all pesticide labels at the time of use for the most current label registration.
Modern technology, fluctuating export markets, changing U.S. Department of Agriculture farm policies and environmental regulations all contribute to soybean growers’ needs for careful planning and management to assure high yields and profitable production.
The publishers and contributors do not assume any responsibility, make any guarantees or offer any warranties in regard to the results obtained from the use of the recommendations appearing in this guide.
Soybean Growth and Development
The soybean is a dicotyledonous plant that has epigeal emergence, meaning that during germination, the cotyledons are pulled through the soil surface by an elongating hypocotyl. The soil-penetrating structure is the hypocotyl arch.
Once emerged (VE stage), the green cotyledons (seed halves) open and supply the new seedling with stored energy while capturing a small amount of light energy. The growing point is between the two cotyledons, and because it is above the ground, it could be killed by a spring frost or physical damage. This is in contrast with corn, wheat, field pea or lentil, in which the growing point is below the surface during the early development stages.
The first true vegetative leaves formed are the unifoliolate leaves. These two single leaves form directly opposite one another above the cotyledonary node (VC stage). All other leaves are trifoliolates and consist of three leaflets (V1-n stages) and have an alternate arrangement on the stem.
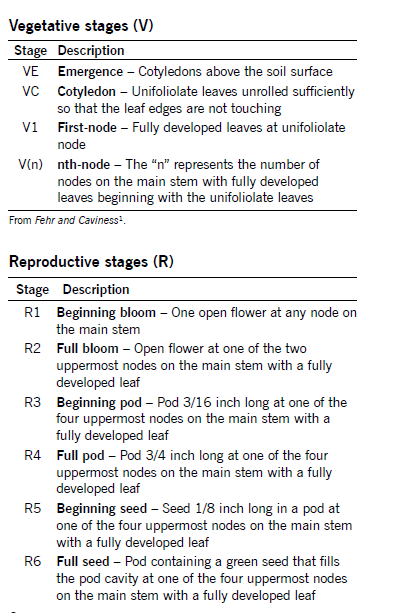
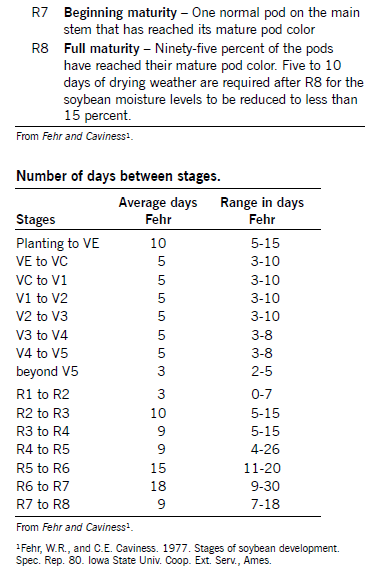
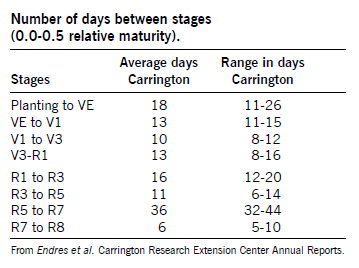
Growth Stages
Soybean development is characterized by two distinct growth phases. The first is the vegetative (V) stages that cover growth from emergence to flowering. The reproductive (R) stages cover growth from flowering through maturation.
Plant stages are determined by classifying leaf, flower, pod and seed development. Staging also requires node identification. A node is the part of the stem where a leaf is (or has been) attached.

Figure 1. Soybean emergence.
A leaf is considered fully developed when the leaf at the node directly above it (the next younger leaf) has expanded enough so that the two lateral edges on each of the leaflets partially have unrolled and are no longer touching.
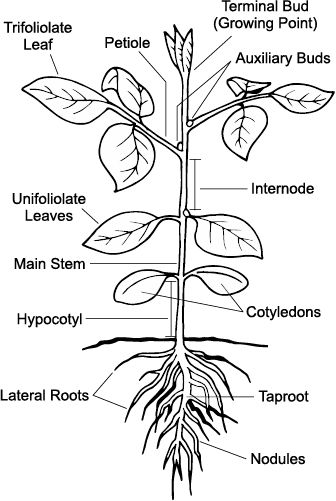
Figure 2. The soybean plant in V2 stage of development.
Variety Selection and Adaptation
Soybean variety selection should be based on maturity, yield, seed quality, lodging resistance, iron-deficiency chlorosis tolerance and disease reaction. Comparative maturity and yield of public and private soybean varieties can be obtained from a current copy of Extension publication A843, “North Dakota Soybean Variety Trial Results and Selection Guide.”
Later-maturing varieties tend to yield more than early maturing varieties when evaluated at the same location. After determining a suitable maturity for the field, comparing yields of varieties that are of similar maturity is important. Although late maturity increases yield potential, later-maturing varieties have more risk than earlier-maturing varieties because an early fall frost may kill a late-maturing variety before the beans have completely filled in the pods, which impacts yield and quality.
Soybean Maturity
Soybean respond to day length (the increase of the night length) and heat units, so the actual calendar date a variety will mature is highly influenced by latitude; each variety has a narrow range of north-to-south adaptation. A model is available at the North Dakota Agricultural Weather Network. It will predict the soybean maturity based on the selected maturity group, planting date and nearest weather station.
Soybean yield and quality are affected if a season-ending freeze occurs before a variety reaches physiological maturity. Dates of maturity are listed in the annual NDSU performance tables and indicate when varieties were physiologically mature. Usually harvest can commence approximately seven to 14 days after the soybean crop is physiologically mature.
Relative maturity ratings are provided for many of the varieties entered in the trials at various locations. Relative maturity ratings for private varieties were provided by the companies entering the variety in the trial.
Varieties of maturity groups 00 (double zero), 0 (zero) and 1 are suitable for eastern North Dakota. Maturity group 00 is very early and primarily is grown in the northern Red River Valley and the north-central area of North Dakota. Maturity group 0 is adapted to most North Dakota counties, while maturity group 1 primarily is suitable for southeastern North Dakota. These maturity groups are further subdivided. For example, a 0.1 maturity group is an early group 0 variety and a 0.9 is a late-maturity group 0 variety.
The best way to select a high-yielding variety is to use data averaged across several locations and years. Because weather conditions are unknown in advance, averaging across several years’ data will identify a variety that likely will yield well across different weather conditions. Selecting a variety that has performed relatively well in dry and moist conditions is the best way to identify a variety that does well, regardless of weather fluctuations.
Phytophthora
Phytophthora root rot caused by the soilborne-fungus Phytophthora sojae is a major disease of soybean in North Dakota. Phytophthora root rot tends to be more of a problem in the Red River Valley (RRV) and on poorly drained, heavy-textured soils, but the disease can cause significant stand reduction and yield loss in other areas when conditions are favorable.
Many varieties have Phytophthora root rot-resistance genes. Each gene for resistance confers resistance to a different race (or races) of Phytophthora. For example, a gene that may confer resistance to Race 3 may not confer resistance to Race 4, and vice versa.
According to a survey of Phytophthora races done by NDSU’s soybean pathologist, Berlin Nelson, Races 3 and 4 are the most common in North Dakota. However, numerous other races are found, especially in the RRV.
The two most common resistance genes found in commercial soybean varieties are Rps 1c and Rps 1k. Unfortunately, numerous races can attack those sources of resistance.
Two better sources of resistance are Rps 3A and Rps 6, and varieties that have two different genes for resistance also are a good choice. Although the use of a soybean variety with resistance to P. sojae does not guarantee control because you will not know what races of P. sojae are in your fields, deploying resistance genes will maximize the likelihood of some protection against Phytophthora root rot.
Iron-deficiency Chlorosis
Iron-deficiency chlorosis (IDC) is a major problem primarily in the eastern part of North Dakota and is caused by iron being less available in soil with a pH greater than 7 and the presence of soil carbonates. Iron-chlorosis symptoms are most common during the two- to seven-trifoliolate leaf stages.
Plants tend to recover and start to turn green again during the flowering and pod-filling stages. However, IDC during the early vegetative stages can reduce yield severely.
Some varieties are more tolerant to IDC than others. For high-pH soils with known IDC problems, select an IDC-tolerant variety of suitable maturity that is high yielding. Data on genetic differences for IDC tolerance is available in publication A-843, “North Dakota Soybean Variety Trial Results and Selection Guide.”
Soybean Cyst Nematode
The soybean cyst nematode (SCN), Heterodera glycines, is a small parasitic roundworm that attacks the roots of soybean plants. Nematodes often are undetected because above-ground symptoms are uncommon until a 15 to 30 percent yield loss has occurred.
Soybean cyst nematode has been confirmed in 19 counties in North Dakota as of 2019. Growers are strongly urged to test their soils for SCN. If a positive sample for SCN is found, growers should begin managing SCN actively.
Crop rotation and resistance are the most important management tools against this disease. Two sources of resistance to SCN — PI88788 and Peking — can be found in varieties suitable for North Dakota. These sources are effective in the vast majority of the soybean fields in the state. However, the level of resistance in each variety is variable, so selecting the most resistant variety possible and monitoring the field for SCN is important.
For SCN management, a rotation out of soybean for two to three years is beneficial. Dry edible beans are susceptible to SCN and should not be used as a rotation crop for managing SCN. Nematicide seed treatments also are available and may help manage SCN; however, they are not a substitute for resistance and rotation.
Specialty Soybean
Food Soybean
Some soybean varieties have been developed for human consumption and have special food-processing characteristics. Tofu is a white curd that primarily is consumed in Asian countries. Special varieties have been developed that are high in protein and make smooth-textured tofu. These high-protein tofu types are lower yielding than the regular varieties that are sold to the elevator.
Natto is another human food product made from soybean. Natto is a fermented product made from whole soybean that is cooked. Natto varieties are very small seeded and tend to yield even less than the specialty varieties developed for the tofu market.
Growers should consult university publications on soybean variety performance to determine how much less these specialty varieties yield, compared with oilseed soybean. Based on the lower yield, a higher price per bushel needs to be obtained to economically justify growing these specialty soybean types. A contract should be arranged prior to growing these special types so that a market will be available.
Oil Modified
Soybean varieties with modified oil content are commercially available. Different fatty acid compositions modify the type of oil the soybean plant produces in the seed. Low saturated fats are desirable because this type of oil is better for human health.
High oleic, low palmitic, low stearic and low linolenic acid content are all genetic modifications that produce more healthful oil for human consumption. We have seen no indication that these modifications reduce yield.
However, yield of specific varieties with modified oil content should be evaluated to determine whether high yield has been incorporated with the modified oil content. These specialty varieties should be marketed as identity-preserved (IP).
Seedbed Preparation
Soybean can be grown on a wide range of soil types under various cultural practices. Because of seed size and physiology, soybean seeds require about 50 percent of the seed weight in moisture to germinate. Also, soybean is planted only 1 to 1½ inches deep. These factors explain why preparation of a firm, uniform seedbed is important for optimum stand establishment.
Many farmers are growing soybean using conservation tillage including no-till. Special planters or drills may be required to handle surface residue in no-till and some reduced-tillage systems.
Soybean, like other legume crops, has difficulty emerging through compacted layers and surface crusts. Soybean is very susceptible to elevated salt levels in the soil and waterlogged conditions.
Planting Date
Soybean is susceptible to frost and prolonged exposure to near-freezing conditions in the spring and fall. Plant soybean after the soil has warmed to 50 F and air temperatures are favorable.
Soybean generally should not be planted earlier than five days before the average last killing frost or projected last frost date for a season. This provides less than a 50 percent chance of frost killing the soybean plant. Early in the season in a no-till or minimum-till situation, the residue tends to retard heat transfer from the soil to the air, which creates a potential for more frost damage to the young soybean plant.
Very early planting in cool, wet soil may result in low germination, increased incidence of seedling diseases and poor stands. Planting dates during the first half of May are favorable for highest yields with a reduced risk of frost injury.
Planting early in the season allows the use of full-season varieties, which typically yield more than shorter-season varieties. Recent research indicates that if conditions are right during the planting season, waiting to plant may reduce the yield by 0.3 bushel per acre per day delay.
Data from NDSU date-of-planting studies indicated that late plantings had lower seed yields, poorer seed quality, lower oil content, shorter plant height and pods set closer to the ground, compared with optimum planting dates. Yield increased 8 percent with first week of May or earlier planting dates when averaged across nine NDSU trials conducted in south- and east-central North Dakota.
Some early maturing varieties have had acceptable yields when weather factors such as hail, late spring frost, or floods necessitate late planting or replanting.
Soybean stands with poor emergence often are replanted without considering the yield-compensating ability of the plants in the initial stand. The yield of an initial planting at less than full stand must be compared to the yield of the replanted crop to determine whether replanting is justified.
Replanting costs include seed, tillage, replanting and labor. The yield of a replanted crop must be sufficiently greater than the yield of the initial planting to cover the expenses associated with replanting. The risk of fall freeze damage to the replanted crop must be considered when deciding the maturity of the variety selected for replanting.
Planting Rate and Depth
Soybean yields have not varied significantly for a wide range of plant populations. An established plant population of approximately 150,000 plants per acre is desirable regardless of row spacing. Averaged across 44 NDSU trials, planting rates of 150,000 to 175,000 pure live seeds (PLS) per acre increased yield by 6 percent, compared with planting rates of 100,000 to 130,000 PLS/acre.
Seeds per pound in available varieties range from 2,200 to 3,400, with an average of 3,000 seeds per pound. High planting rates may cause yields to decrease in low-rainfall environments because of drought stress, and in a good rainfall year, high planting populations may lodge more than low populations. Low plant populations reduce lodging but contribute to low pod set and excessive branching.
An extremely low seed number per foot of row may result in erratic stands due to a lack of seedling energy necessary to break the soil surface. This may be critical in solid-seeded stands in which soils are prone to crusting.
Planting rates should be increased (around 10 percent) to compensate for naturally occurring factors that cause some live seeds not to become established plants. Slightly higher planting rates also may be advantageous with late planting dates or in no-till, where soil temperatures generally are lower.
If planting in narrow row spacings (less than 10 inches), we suggest that soybean planting rates be adjusted upward. Planting rates of 175,000 seeds per acre in 12- to 15- inch row spacings and 200,000 seeds per acre when drill seeding are recommended.
To ensure planting enough soybean seed, the planting rate should be based on a seed count. You will need to know the following to calculate the rate:
1. Desired established plant population
2. Average stand loss for your farm
3. Germination value of your seed
4. Number of seeds per pound of seed
The following is an example for calculating planting rate
1. Desired established plant population is 150,000 plants per acre
2. Normal stand loss is 10 percent
3. Seed germination is 95 percent
4. Soybean seed has a seed count of 3,000 seeds per pound, or 180,000 seeds per bushel
The planting rate (PR), expressed as the number of seeds per acre can be calculated from the following equation: PR = D*[100/(M1)]*[100/(100-M2)], where D is the desired plant density per acre 150,000, M1 (germination percent = 95 percent) and M2 (average percent stand loss on the farm = 10 percent).
PR = 150,000*[100/(95)]*[100/(100-10)] = 175,450 seeds per acre
175,450 seeds ÷ 3,000 seeds per pound = 58.5 pounds/acre (lb/a) of soybean seed needs to be planted.
Plan to cover seed 1 to 1½ inches deep and place the seed in moist soil. Planting deeper than 2 inches or in a soil that crusts may result in poor emergence and plant stand.
Row Spacing
Midwest research demonstrates that higher yields of soybean can be obtained in rows less than 30-inch spacing if stands are well-established and weeds are controlled adequately. NDSU research indicates that 14- to 22-inch row soybean outyield wider-spaced (28- to 30-inch) soybean by an average of 4 percent when averaged across 24 trials.
The advantages of narrow-row soybean are increased yield, reduced soil erosion, increased harvesting efficiency, and early crop canopy closure to help conserve soil moisture and suppress weeds. Planting in wider rows provides the opportunity to use row-crop planters, permits cultivation for weed control and may reduce the risk of white mold (sclerotinia).
Planting Guide
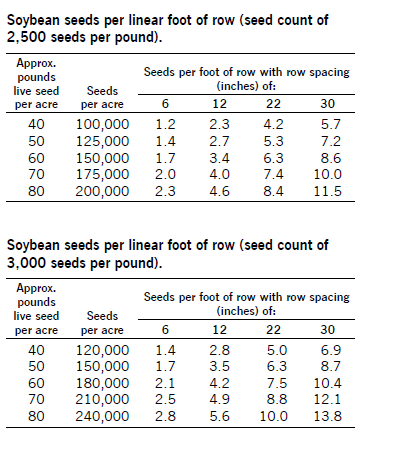
To determine the number of seeds per acre planted, add seed to your planter or drill and operate it on a firm soil surface so seed is visible on the surface. Operate it for a short distance close to your normal operating speed. Then go back and count the number of seeds dropped in 1 linear foot of planter row.
Make several counts and determine an average. Refer to one of the following charts to see that you are planting the number of seeds that you calculated in the earlier section.
Air Seeder Calibration
Calibrating an air seeder usually is done by following the directions listed in the operators manual. It usually will tell you to hand turn the seed metering system a number of turns for a predetermined area. This often is listed for 1/10 or ¼ acre.
Then the metered seed needs to be weighed on a scale. Sometimes these scales are provided with the air seeder. The weights need to be multiplied by 10 for 1/10 acre or multiplied by 4 for ¼ acre, and then adjustments can be made based on the previous calculated amounts.
Another method for calibrating an air seeder requires collecting seed from the seed openers. Probably the easiest method is to place a tarp under the openers, collect seed over an area or distance (1/10 acre) and weigh the pounds of seed collected.
First, determine the pounds of seed to plant as calculated in the planting rate section of this publication. Then (1) determine the circumference (ft.) of the seed meter drive wheel on your seeder using the following formula:
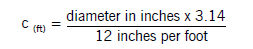
(2) Determine the drive wheel revolutions required to equal 1/10 acre. Use the following chart to calculate this number, which is based on the width of your air seeder.
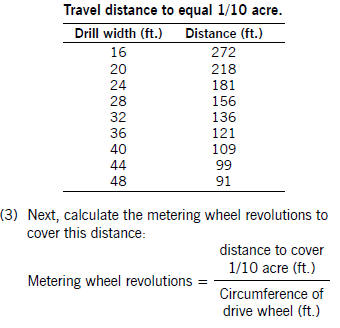
(5) Weigh the seed collected on the tarp and multiply times 10. This number should equal the pounds of seed you want to plant.
Drill calibration is becoming extremely important so you can be sure you are planting the correct amount of seed. If the amount of seed determined with either method is not equal to the amount of seed you desire, make an adjustment to the feed rate and recheck your seeder. This method also works for determining the pounds of fertilizer to be applied.
Soybean Soil Fertility
Dave Franzen, Extension Soil Science Specialist
Soybean has a need for 14 mineral nutrients: nitrogen (N), phosphorus (P), potassium (K), sulfur (S), calcium (Ca), magnesium (Mg), zinc (Zn), manganese (Mn), copper (Cu), iron (Fe), boron (B), chloride (Cl), nickel (Ni) and molybdenum (Mo). Of these, North Dakota soils provide adequate amounts to soybean except for N, P, K, S and Fe.
Nitrogen Fixation
Although the atmosphere is 78 percent nitrogen gas, plants cannot use it directly. Plants can use only ammonium-N or nitrate-N.
Soybean is a legume and normally should provide itself N through a symbiotic relationship with N-fixing bacteria of the species Bradyrhizobium japonicum. In this symbiotic relationship, carbohydrates and minerals are supplied to the bacteria by the plant, and the bacteria transform nitrogen gas from the atmosphere into ammonium-N for use by the plant.
The process of soybean infection by N-fixing bacteria and symbiotic N fixation is a complex process between the bacteria and the plant. The right species of N-fixing bacteria must be present in the soil, either through inoculation of the seed or the seed zone at planting.
N-fixing bacteria are attracted to soybean roots by chemical signals from the soybean root in the form of flavonoid compounds (1). Once in contact with the root hairs, a root compound binds the bacteria to the root hair cell wall. The bacteria releases a chemical that causes curling and cracking of the root hair, allowing the bacteria to invade the interior of the cells and begin to change the plant cell structure to form nodules (See photo section).
The bacteria live in compartments, up to 10,000 in each nodule, called bacteroids (Figure 3). Each bacteroid is bathed in nutrients from the host plant, and the bacteroid takes nitrogen gas from the soil air and converts it to ammonium-N using the enzyme nitrogenase, which consists of one Fe-Mo (iron-molybdenum)-based protein and two Fe (iron)-based proteins. In this region, iron deficiency chlorosis (IDC) may result in poor nodulation and may contribute to N deficiency as well as iron deficiency.
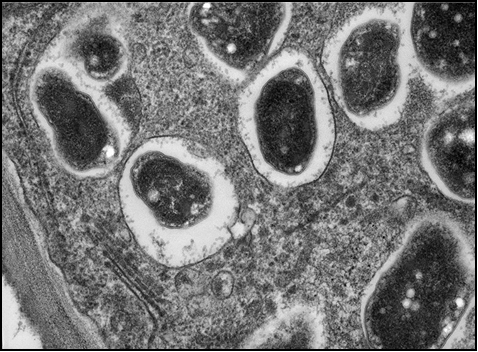
Figure 3. Soybean nodule cross-section micrograph showing individual bacteroids.
(Louisa Howard, Dartmouth College; used with permission)
Using Inoculants
If soybean will be planted in a field for the first time, the seed will need to be inoculated with Bradyrhizobium japonicum (soybean inoculum). Several inoculum types can be used: peat-based, liquid-based or granular.
Of the three, granular appears to be the most foolproof for a first inoculation. The other two also can be used, but the frequency of mistakes is much higher. No formulation is free of error.
For the peat-based and liquid-based treatments, all seed should have inoculum attached to it when it enters the soil. Peat-based inoculants can vibrate off the seed if they are not applied with an adequate sticking agent. Application of liquids could be poorly calibrated and inoculum may not hit all the seed if the application is not made carefully. Even granular will have problems in performing if the seeder is poorly calibrated.
If you use proper care in handling and application, the success rate of all inoculation is very high. In the rare event that nodulation does not take place, supplemental N will have to be applied to reach yield potential. In-season foliar N application is not recommended, and slow-release liquid N sources have no higher foliar N efficiency, compared with UAN (urea-ammonium nitrate) solutions.
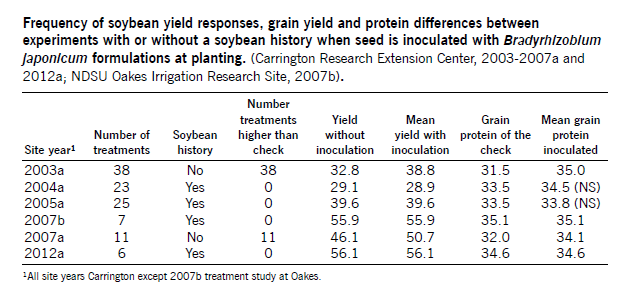
If a field has been seeded to soybean previously and nodulation was effective, you have only a small chance that inoculating again will be economically effective (See table on page 27). Average across 16 NDSU trials, seed yield increased 2 percent with inoculated seed on fields with previous soybean production history, compared to non-inoculated seed.
In the region, soybean grown in soils with conditions that did not support iron deficiency chlorosis sometimes responded to higher soil nitrate levels. However, higher soil nitrate levels in soils increase the severity of IDC in soils where IDC supporting conditions prevail.
Nitrogen is not required by soybean if adequate inoculation is present. In North Dakota experiments, the only financial benefits to supplemental N have been to first-year soybean in which initial inoculation resulted in poor nodulation.
Nutrients
Nitrogen Recommendations for Soybean
Nitrogen is not recommended for soybean, even first-year soybean. Elevated soil nitrate may increase the likelihood and severity of iron-deficiency chlorosis. The economics of late-season N application do not justify application.
Phosphorus
Soybean respond better to broadcast applications of P than to banded applications with or near the seed. Several recent studies confirm that broadcast application of P is safer than in-furrow P and, therefore, more likely to provide the desired yield respond, compared with seed placement.
If soil test levels are low to very low (less than 8 parts per million [ppm]; Olsen P), then a separate application of broadcast P is justified. However, if soil test levels are medium or higher, the level of soybean’s response to P fertilizer is small, not justifying a separate P application. Soybean roots are excellent scavengers of P at medium or higher soil test levels.
Even though a broadcast application of P may result in several more bushels of soybean than a banded application, some producers will elect to apply P with the seed. No fertilizer of any kind is recommended with soybean seed in a 15-inch row or wider. However, using a double-disk drill with 6-inch spacings, up to 10 pounds of N per acre may be applied to soybean as a P fertilizer (do not use urea) without yield reduction. However, considering that the goal of any fertilizer application is to produce income, seed-banding research has shown limited economic success.
With air seeders, the risk to soybean plants with fertilizer spread across the seed zone will be decreased. Even though applying up to 10 pounds of N per acre with a 6-inch row spacing is possible, dry weather at planting will increase the risk of injury
Therefore, producers should not to push rates too hard toward the limit because of the variability within fields in sand and soil water content. Sandier textures and low available soil water soils may show more stand injury than other areas of the field. The best recommendation for P application is to broadcast it.
Potassium
Testing the soil for K and referring to the clay chemistry map of North Dakota for guidance in K fertilization are important. After many years of soybean production, some soils may have been mined of K.
Sandier textured soils in the beach ridges west or east of the Red River Valley have been low in K for many years. Some sandier hilltops in the glacial till plain or in residual materials west of the Missouri River also may be lower in K. Some limited soil testing based on general landscape will show whether K is needed in these areas.
Generally, coarser-textured soils are more at risk for K deficiency than heavier soils. However, with continuous soybean production, even heavy soils may be at risk of being deficient.
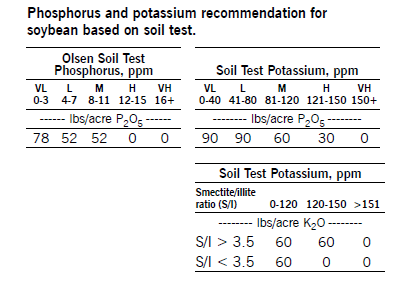
Potassium should be broadcast or banded, with the seed and fertilizer separated. Do not apply K fertilizers with the seed. If the soil test is less than 150 ppm in soils with a smectite/illite ratio greater than 3.5, application of 60 pounds per acre of K2O is recommended to prevent K deficiencies in a dry year. If the soil test is less than 120 ppm in soils with a smectite/illite ration less than 3.5, 60 pounds per acre K2O is recommended.
Phosphorus and potassium recommendation for soybean based on soil test.
Sulfur
Sulfur deficiency has become common in corn and small grains in North Dakota, and canola is always at risk for S deficiency. Sulfur deficiency in soybean is rarer.
Soybean growing on sandy soils (loamy sands, sandy loams with low organic matter) are most at risk, particularly in a wet spring, but the overall risk even under these conditions still is low. Your most profitable option might be to observe soybean leaf color through the season and apply S to areas of the field that show S deficiency into the season.
Soil pH
Soybean plants grow best around a pH of 6.5. Lowering the pH from 8 to 6.5 is not an option because of the cost of amendments and the formation of salt if the application is successful. However, low pH values are common in fields in North Dakota.
Sampling by landscape position reveals pH patterns, whereas composite soil sampling does not. Application of ground limestone or sugar beet lime would be justified if the soil pH is lower than 6.
Zinc
Soybean is not sensitive to low soil Zn levels in North Dakota. Soybean grows well at Zn soil test values much lower than North Dakota Zn-sensitive crops, which are dry bean, corn, flax and potato.
Iron
North Dakota soils have about 5 percent of iron (Fe) by weight. However, only a tiny fraction ever is available to plants.
Iron in well water is reduced iron (Fe++ or Ferrous iron). Ferrous iron is very soluble in water. The weight of a No. 2 carpenter nail can be dissolved in water if it was ferrous iron.
Unfortunately, as soon as ferrous iron is exposed to oxygen, it oxidizes to oxidized Fe (Fe+++ or Ferric iron). Ferric iron is a trillion times less soluble than ferrous iron. Plants, except for aquatic plants such as rice and pondweed, implement Fe uptake strategies to improve Fe nutrition and avoid deficiency.
In soybean, Fe is mobile in the plant from germination through the first mono-foliolate leaf. As the first trifoliolate leaf emerges, Fe becomes immobile in the plant and must be taken up continually through the season to avoid deficiency.
The soybean strategy for Fe uptake begins by soybean roots acidifying the soil environment directly around the soybean root. The acid soil environment is necessary for the activity of an Fe-reducing protein that is secreted by the soybean root. If the root remains acidic, the Fe-reducing protein contacts oxidized iron and reduces it to soluble ferrous iron, making it available to the plant.
In soils that are susceptible to iron deficiency chlorosis, the causal soil condition is the presence of carbonates (CO3--). As the soil becomes wetter, the solubility of carbonates increases, producing bicarbonate (HCO3-). Bicarbonate neutralizes the acidity around plant roots and makes the Fe-reducing protein secreted by the roots ineffective.
Iron foliar sprays are generally not effective in correcting a deficiency. The best application to reduce IDC is ortho-ortho-EDDHA Fe chelate applied with water in-furrow at planting. The ortho-ortho-EDDHA not only succeeds in delivering Fe to the plant root early in the season, but after conveying its original Fe, it has the ability to go back into the soil solution, grab additional Fe and deliver it to the plant root with the soil water stream.
The amount of ortho-ortho EDDHA in relation to ortho-para EDDA (Figure 4) is very important. Recent research at NDSU has shown that the response of soybean to EDDHA fertilizer is directly proportional to the percentage of ortho-ortho EDDHA Fe.
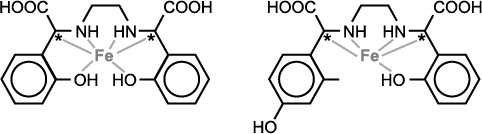
Figure 4. Ortho-ortho EDDHA (left); ortho-para EDDHA (right).
An effective IDC prevention strategy should not rely on the application of ortho-ortho-EDDHA alone, but on a comprehensive approach to the condition. An IDC-tolerant variety should be selected. A four-state study led by NDSU found that the highest yield for a soybean field with soils with and without susceptibility to soybean IDC would be best managed by planting a high-yielding, IDC-intolerant variety in non-IDC soils and an IDC tolerant-variety in the IDC-susceptible soils.
The main management factors in managing IDC are selecting a suitable field and a tolerant variety. However, if a tolerant variety is not enough to reduce IDC pressure, soybean could be planted in wide rows (30-inch) and/or at higher than normal planting rates. The causes of the increased plant density reducing IDC could be related to reduced soil moisture under the row, higher root-zone acidity that would favor activity of the Fe-reducing substance secreted by the soybean root, or other unidentified mechanisms.
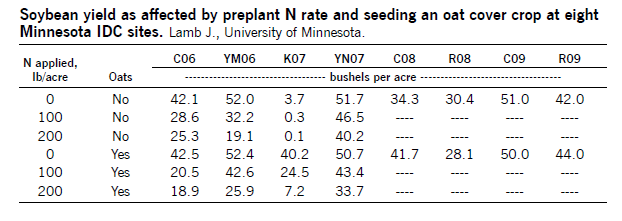
A three-state study found that seeding a cover crop at 1 bushel per acre of oats or other easily killed small-grain cover crop about the day of soybean planting can reduce excess water and take up some excess soil N. Depending on soil moisture, the oats may be killed with herbicide early if conditions are dry, or up to the five-leaf stage of oats if the season is wet. The use of an oat cover crop resulted in as high as 40 bushels per acre more soybean where oats were used, compared with where they were not at a Minnesota site in a wet season.
Because soil salinity aggravates and increases the severity of IDC, a comprehensive, rotation-based strategy should be imposed to reduce soil salinity as much as possible. Management strategies include selection of better salinity-tolerant crops; the use of alfalfa strips to reduce roadside salinity; the use of alfalfa above saline seeps to reduce the severity of the seep; the use of cover crops when possible before, during or after cropping to reduce field water table; and possibly tile drainage if possible, practical and socially and/or regulation permissible.
Soybean Weed Control
Joseph Ikley, Extension Weed Specialist
The weed control suggestions in this production guide are based on the assumption that all herbicides mentioned will have a registered label with the Environmental Protection Agency. Soybean treated with a nonregistered herbicide may have an illegal residue which, if detected, could cause condemnation of the crop.
Federal law makes liable for seizure any raw agricultural commodity that possesses a pesticide residue for which no exemption or tolerance has been established or that exceeds the tolerances established by the Food and Drug Administration. People using herbicides in a manner contrary to label instructions are subject to penalty under federal and state laws.
North Dakota State University or its officers or employees makes no claims or representations that the chemicals discussed will or will not result in residues on agricultural commodities and assumes no responsibility for results from using herbicides.
Instructions for the registered uses of herbicides are given on container labels. Read and follow label instructions carefully. Use pesticides only as labeled.
Herbicide labels also can be found on the web.
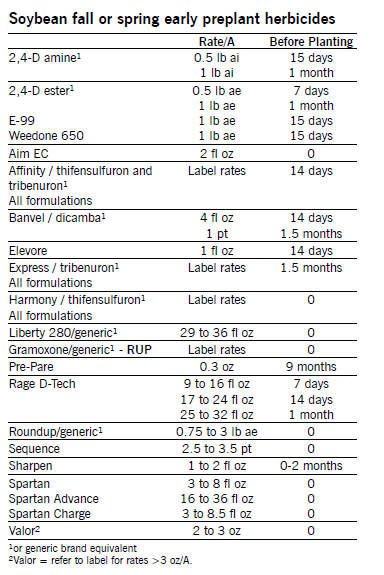
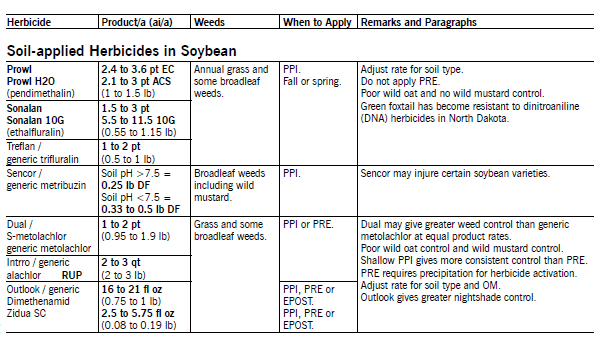
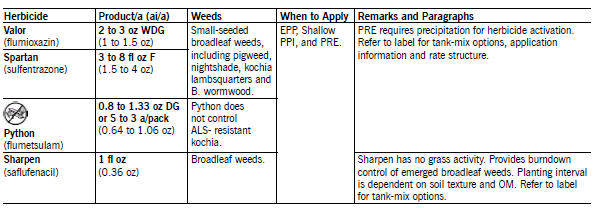
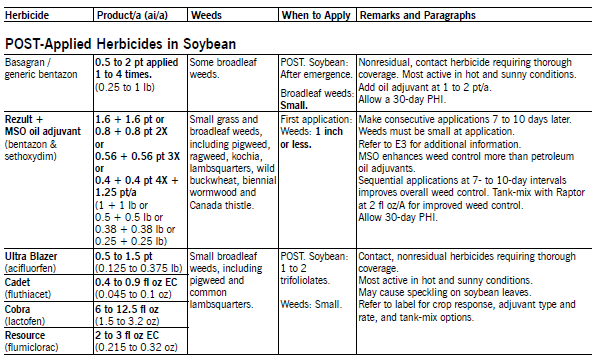
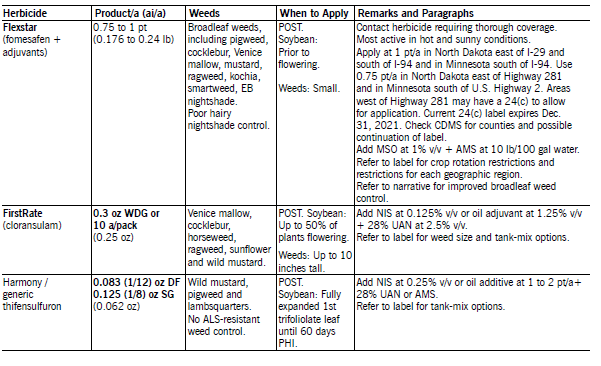
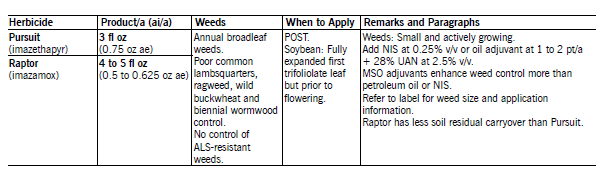
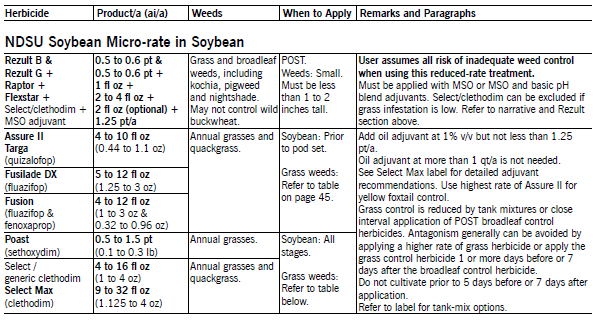
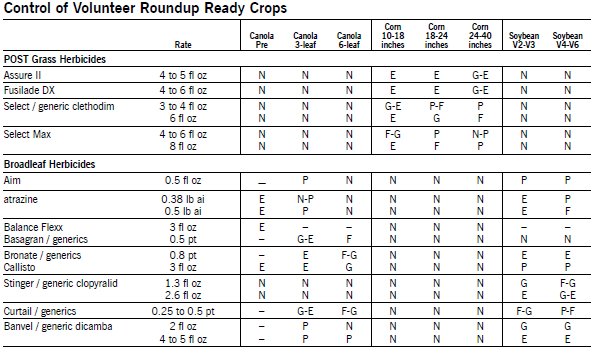
Weed Management in Roundup Ready Soybean
NDSU recommends using herbicides with different modes of action and different weed control management practices in Roundup Ready soybean production to delay development of glyphosate-resistant weeds.
COMMANDMENT 1 — Control weeds before 2 to 4 inches tall to avoid yield loss.
Remove weeds early, especially when grass weed populations are high. Some data from the Midwest indicate that soybean yield may not be reduced by delaying Roundup/generic glyphosate application until weeds are up to 6 inches tall. However, data from the northern Plains show that, especially under dry conditions, soybean yield loss will occur if weeds become greater than 4 inches tall prior to Roundup/glyphosate application.
Roundup/glyphosate at 1.5 oz ae/a controls foxtail, 2.25 oz ae/a controls volunteer small grain, and 3 oz ae/A controls wild oat and downy brome. Use higher rates on broadleaf weeds, larger weeds and tolerant weeds, or if weeds are under environmental stress.
Three Systems of Weed Control in RR Soybean
1. PRE followed by glyphosate POST: All PRE herbicides require rain for activation.
Tables lists many registered PRE soybean herbicides that can be used in herbicide-resistant soybean. PRE herbicides at 2/3 to the full labeled rate may give 60 to 99 percent control of some grass and broadleaf weeds, will reduce weed infestations emerging with soybean, will allow more flexibility in application of POST herbicides and will help protect yield from early season weed competition.
2. Roundup/generic glyphosate + POST broadleaf herbicide (different mode of action):
Several herbicides listed in the following table will improve control of weeds not controlled by Roundup/glyphosate. Roundup/glyphosate has no soil residual and will not control weeds emerging after application. Roundup/glyphosate may not control some weed species or biotypes. Many POST herbicides listed will give residual weed control. Follow label directions for tank-mix and application information.
3. Roundup/generic glyphosate (EPOST = 2- to 4-inch-tall weeds) followed by Roundup/glyphosate (POST = 14 to 21 days later):
This program will increase the risk of weed resistance unless other strategies are used in rotational crops.
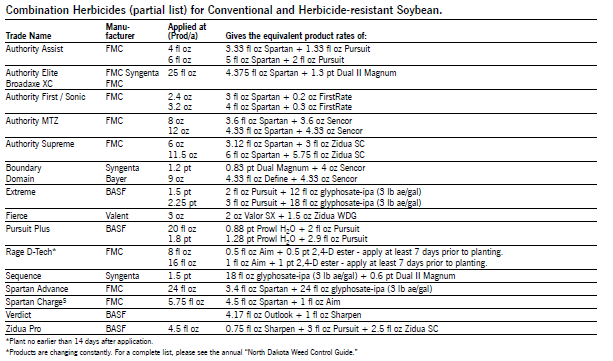
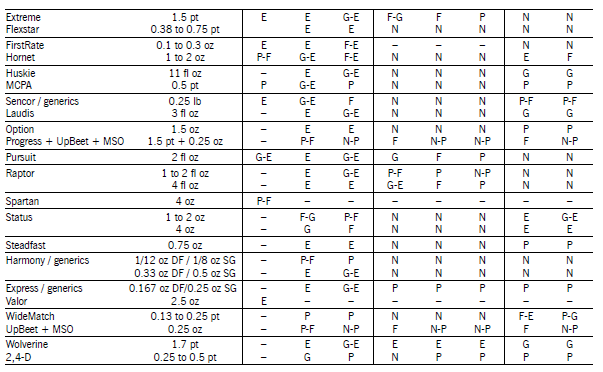
The following table shows herbicides to apply in tank-mix or sequentially with Roundup/glyphosate in RR soybean for control of weeds not controlled by Roundup/glyphosate. Weed ratings are control without Roundup/glyphosate. Refer to the label for tank-mix and specific application information. Residual weed control listed in the table refers to control of subsequent flushes of weeds after herbicide application.
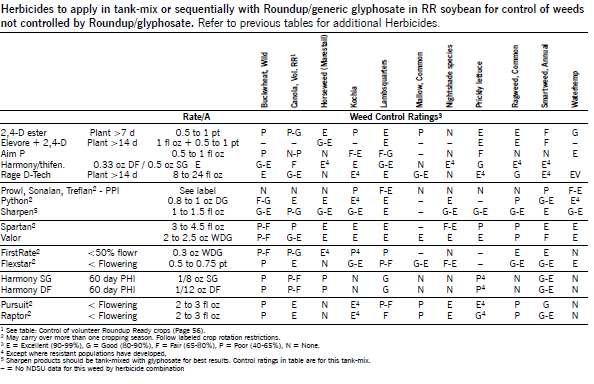

Herbicide Comments
Soybean is a poor competitor with weeds when cool soil temperatures cause slow germination and growth, but soybean does compete effectively in warm soils when germination and growth are rapid. Soybean production requires good cultural practices. Prepare the seedbed prior to planting to kill germinating weeds.
Management practices such as thorough seedbed preparation, adequate soil fertility, the choice of a well adapted variety and the use of good-quality seed all contribute to conditions of good competition with weeds. A rotary hoe or harrow may be used to control weeds after planting but before the soybean emerge or after emergence when soybean are in the one- to two-trifoliolate leaf stage. A rotary hoe or harrow helps activate PRE herbicides under dry conditions and increase weed control.
The rotary hoe is an effective and economical weed control method when a field is not trashy, lumpy or wet, and when weeds are emerging. Cultivation is most effective when soybean are slightly wilted during the warm part of the day because the crop is less susceptible to breakage and weeds will desiccate quickly.
Poast (sethoxydim) plus petroleum oil adjuvant or applied POST controls annual grasses. Assure II (quizalofop), clethodim, Fusilade DX (fluazifop P), Fusion (fluazifop-P & fenoxaprop-P) plus petroleum oil adjuvant or Select Max (clethodim) applied POST controls annual grasses and quackgrass. Methylated seed oils (MSO) have performed equally to petroleum-based oil additives. Refer to the Select Max label for adjuvant information.
Re-treat quackgrass when regrowth is 4 to 8 inches tall. Poast only suppresses quackgrass. Most broadleaf herbicides tank-mixed with POST grass herbicides often will reduce grass control, compared with the grass herbicide applied alone. Reduced grass control can be avoided by applying the grass herbicide at least one day before or seven days after application of a broadleaf herbicide.
Assure II may provide excellent green foxtail control but less yellow foxtail control. Lower yellow foxtail control may result from applying Assure II at reduced rates, with broadleaf herbicides, or to large or stressed plants. The addition of fertilizer may enhance yellow foxtail control and control of stressed grasses.
Clethodim is an ACCase mode of action herbicide, similar to Assure II, Fusilade and Poast. However, in NDSU research, clethodim controls many grasses documented resistant to other ACCase herbicides and is antagonized less by tank-mixes with broadleaf herbicides. We recommend that clethodim be used in rotation with herbicides of different modes of action and in a resistant weed management program.
Several generic brands of clethodim are available, but not all formulations are identical to the original Select formulation. Select, Clethodim, Trigger and Volunteer are the same, but Arrow, Prism, Section and Select Max all have different formulations. Select Max is a 1 lb/gal formulation, contains activating adjuvants in the formulation, and allows the use of NIS, PO or MSO, depending on the tank-mix partner.
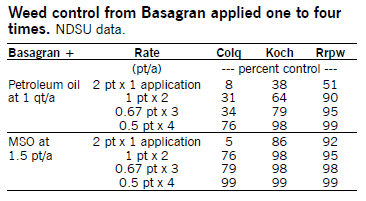
Basagran (bentazon) at 0.5 to 1 qt/a applied POST controls many annual broadleaf weeds and suppresses Canada thistle. NDSU research has shown greater broadleaf weed control, especially in kochia, lambsquarters, redroot pigweed and wild buckwheat, by applying Basagran as split treatments twice each at 1 pt/a, three times each at 0.67 pt/a or four times each at 0.5 pt/a, compared with one application at 2 pt/a. Make applications seven to 10 days apart, depending on the weed growth rate, growing conditions, size of weeds at application, degree of weed control from the first application and sequential flushes. The first application must be made to small weeds (1 inch).
For Canada thistle control, apply Basagran at 1 qt/a when plants are 8 inches tall to bud stage and make a second application at 1 qt/a seven to 10 days later.
The NDSU Soybean Micro-rate concept is based on the Sugareet Micro-rate and substitutes additional weed management for reduced herbicide rates. Application to small weeds is essential for success. The micro-rate can be applied more than once in dry beans to control emerging weed flushes, but applying a foundation herbicide treatment (DNA or acetanilide) may require only one POST application. MSO adjuvant is required for optimum weed control. The POST grass herbicide can be excluded if grass populations are low.
Preliminary data show weed control can be improved by increasing spray volume. The first application can be made at 10 gpa when weeds are small and less than 3 inches tall. Increase the spray volume by 10 gpa for every 3 inches in weed height. The addition of AMS at 1 lb/a also increases weed control.
Weed control from the micro-rate is best when temperature plus humidity is greater than 140. Increasing spray volume and using AMS may help improve weed control when the value is below 140.
Sequential micro-rate applications will provide greater broadleaf weed control than from a single application at full rates and can be used in all crops where Basagran is labeled. Apply with an oil additive at 1qt/a (1pt/a by air). Do not reduce the amount of oil adjuvant with the micro-rate. MSO adjuvant has shown greater enhancement of Basagran than petroleum oil (COC) adjuvants, but the cost of MSO is higher.
Basagran is safe to soybean at all stages. The total maximum seasonal use rate is 4 pt/a, so the rate of the micro-rate can be increased if weeds are large at application or if sequential applications are delayed due to rain or wind.
Basagran commonly is combined with fertilizer micronutrients that may cause incompatibility problems resulting in zinc precipitation. Chelated zinc materials (which are black) have greater incompatibility problems than unchelated material (clear). Recommendations to prevent precipitation are to fill the sprayer with water, add Basagran and thoroughly agitate, then add zinc fertilizer material.
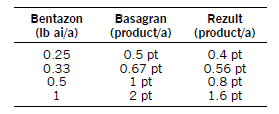
Rezult B and Rezult G (bentazon and sethoxydim) applied POST at equal product amounts controls some grass and broadleaf weeds. Apply with oil adjuvants at 1 to 2 pt/a. Refer to the label or narrative for tank-mix options. Rezult is priced economically, compared with other POST herbicide programs. Rezult may be more economical than Basagran for grass and broadleaf weed control. If so, use the following chart.
Flexstar (fomesafen + adjuvants) applied POST controls many small broadleaf weeds. Apply with NIS at 0.25 to 0.5% v/v or oil adjuvant at 0.5 to 1% v/v. Oil adjuvant increase weed control but also increase the risk of soybean injury. NDSU research has shown good to excellent kochia control when Flexstar is applied at high spray volumes (greater than 17 gpa) with oil adjuvants (especially MSO type) at labeled rates and to kochia less than 2 inches tall.
Soybean injury may result when Flexstar is tank-mixed with EC formulation herbicides that act as an additional oil adjuvant. The activity of fomesafen and the risk of crop injury increase as temperature and humidity increase. A maximum of 0.75 pt/a is allowed in most of North Dakota, while 1 pt/a is allowed through the Midwest. The reduced fomesafen rate reduces carryover and crop rotation restrictions.
Flexstar is labeled on soybean and Reflex is labeled on dry bean. Flexstar contains adjuvants lacking in the Reflex formulation. Reflex may give less consistent weed control than Flexstar and will require better management strategies to achieve adequate weed control. See the label or crop rotation restriction section for additional information.
Alachlor, dimethenamid, metolachlor or S-metolachlor and pyroxasulfone applied PPI or PRE control annual grass and some broad-leaf weeds and do not control wild oat. Apply the higher rate on clay soils high in organic matter. Soybean has good tolerance, and incorporation improves consistency of weed control. Dual products may be surface-applied or incorporated in the fall after Oct. 15 but before the ground freezes or applied in the spring.
Metribuzin controls some annual broadleaf weeds, including wild mustard. Adjust the rate according to the soil type, pH and percentage of organic matter. Some soybean varieties are susceptible to metribuzin; consult seed companies for a list of susceptible varieties. Soybean injury can be reduced by using herbicide combinations with lower rates of metribuzin.
Pursuit (imazethapyr) applied POST controls or suppresses many broadleaf weeds, except ALS-resistant weeds. Pursuit has controlled marshelder, Russian thistle, common cocklebur, sunflower, smartweed and lanceleaf sage in NDSU field trials. Pursuit may not control Venice mallow, horseweed, wild buckwheat, lambsquarters and common ragweed. POST application may not provide adequate soil residual to control subsequent flushes of nightshade due to plant foliage intercepting most of the spray.
However, even a small amount of Pursuit may give a reduction in number and intensity of flushes of other weeds. Pursuit is enhanced greatest by MSO (1.5 pt/A) and basic pH blend (1% v/v) adjuvants. UAN fertilizer (a solution of urea and ammonium nitrate in water) improves weed control, especially lambsquarters.
Crop injury may result if Pursuit or thifensulfuron is applied sequentially or tank-mixed together. In sequential application, the first herbicide reduces the ability of soybean to metabolize the second herbicide. Weeds not controlled by the first herbicide may not be controlled after the second herbicide is applied.
This is particularly important for lambsquarters. Weeds that escape control from the first herbicide may be larger than labeled size by the time soybean can be treated safely with the second herbicide. Delay cultivation for 14 days after application to avoid reduction in weed control.
Tank-mixtures of Pursuit with Assure II, clethodim or Fusilade DX may result in reduced grass control. Reduced grass control can be avoided by applying the POST grass herbicide one or more days prior to or seven days after Pursuit.
Pursuit Plus (imazethapyr and pendimethalin) at 1.8 pt/a applied PPI controls most annual grass and broadleaf weeds, including wild buckwheat. North Dakota state labeling allows its use in North Dakota only south of state Highway 2 at a reduced rate of 1.8 pt/a, which is 75 percent of the full labeled rate.
Pursuit Plus at 1.8 pt/a contains the equivalent of Pursuit at 3 fl oz/a plus 1.75 pt/a of Prowl EC. Add additional pendimethalin at 1.75 pt/a for more consistent weed control. Thoroughly incorporate it into the top 1 to 2 inches of soil. Refer to paragraphs on Pursuit and Prowl for additional information on use and restrictions.
Python (flumetsulam) applied PPI or PRE will control many annual small-seeded broadleaf weeds in soybean. Python does not control large-seeded broadleaf weeds such as common and giant ragweed and common cocklebur. Python requires soil water for optimum weed control. Python also is strongly affected by soil pH. High soil pH increases herbicide activity and the speed of herbicide degrada-tion but also increases the risk of crop injury.
Excellent broad-spectrum weed control may occur when applied on soils with a pH above 7.5, when significant precipitation occurs after application, when rates are based on soil texture and organic matter content, and under light to moderate weed infestations. Some stunting may occur under poor growing conditions on soils with a pH greater than 8.
Raptor (imazamox) applied POST controls nearly all annual grass and broadleaf weeds in soybean except wild buckwheat, lambsquarters, common and giant ragweed, Venice mallow, horseweed, biennial wormwood and ALS-resistant weeds. In NDSU field trails, Raptor has controlled marshelder, Russian thistle and lanceleaf sage less than 1 inch tall. Soil residue of Raptor will not control late-germinating weeds or weed flushes later in the growing season after rain events. Raptor has greater grass and broadleaf weed control, provides improved lambsquarters control and has less carryover and crop rotation restrictions that Pursuit.
Apply Raptor with a basic pH blend adjuvant at 1% v/v or MSO-type adjuvants at 1.25 pt/A. Alternatively, apply with NIS at 0.125 to 0.25% v/v or oil concentrate at 0.5% v/v plus 28% UAN liquid fertilizer at 4% v/v. The use of 28 percent UAN improves control of some weeds such as lambsquarters. MSO-type oil additives should be used when weeds are large and/or stressed.
MSO or basic pH blend adjuvants enhance weed control more than NIS or some petroleum oil additives with or without 28 percent UAN. However, Raptor applied with MSO + UAN may result in crop injury at temperatures greater than 88 F and relative humidity greater than 80 percent.
Refer to the label and paragraph on Pursuit and Raptor for information and restrictions when applying Raptor before or after thifensulfuron or tank-mixing with thifensulfuron or other POST grass herbicides. Crop rotation restrictions are less with Raptor than Pursuit.
However, like Pursuit, Raptor carryover is affected by soil pH. As the soil pH increases, rate of Raptor degradation increases. At a soil pH less than 6.5, the rate of breakdown is slow and injury to sugarbeet and other sensitive crops may occur if planted before the allowed time interval. See the label or information on crop rotation restrictions.
Sonalan (ethalfluralin), trifluralin or Prowl/H20 (pendimethalin) applied PPI controls most annual grasses and some small-seeded broadleaf weeds but provides no wild mustard, common cocklebur and sunflower control. Requirements for proper timing and depth of incorporation differ for each herbicide. Adjust the rate according to the soil type.
Trifluralin must be incorporated in the top 2 to 3 inches of soil within 24 hours of application. Trifluralin incorporation may be delayed up to two days if applied to a cool, dry soil. Incorporation of Sonalan 10G can delayed three to five days after application. Herbicides can be applied with most soil PPI herbicides labeled in soybean. Sonalan has less soil residue than trifluralin or Prowl and may be more active at comparable rates.
Spartan (sulfentrazone) applied shallow PPI or PRE controls most annual small-seeded broadleaf weeds and may control wild buckwheat, marshelder, wild mustard, common ragweed, hairy nightshade, Venice mallow and foxtail partially but provides no perennial weed control. The rate must be adjusted for soil texture, soil pH and organic matter content. Apply 3 to 6 fl oz/a for coarse and medium-textured soils and 4 to 8 fl oz/a for fine-textured soils. Herbicide solubility, activity and phytotoxicity increase as the soil pH increases. The user must read and follow the label for rate information to ensure adequate weed control.
Spartan provides excellent burndown weed control and may be applied up to 30 days prior to planting, but use the higher rate in the appropriate rate range. Spartan can be tank-mixed with most PPI/PRE herbicides registered in soybean.
NDSU research has shown that consistent control of susceptible broadleaf weeds and suppression of foxtail and marginally susceptible broadleaf weeds depends on at least 0.5 to 0.75 inch of rainfall shortly after application and before weeds emerge. Spartan will leave a residue in the soil for more than one year. Refer to the label for crop rotation restrictions.
Harmony GT (thifensulfuron) has activity on wild mustard, lambsquarters, pigweed species, annual smartweed and wild buckwheat. Apply with NIS at 0.125 to 0.25% v/v or oil adjuvants at 0.5% v/v plus liquid fertilizer at 4% v/v. Do not apply with oil adjuvants when tank-mixing with any other herbicide or severe crop injury may occur. See the label or Pursuit paragraph for precautions when tank-mixing with Pursuit and other herbicides.
Thifensulfuron as spray drift or sprayer contamination may cause severe injury to susceptible crops such as sugarbeet and sunflower. Thoroughly clean the sprayer to prevent contamination of subsequent spray mixtures and injury to susceptible crops. Follow the label for improved cleanout procedure.
Valor (flumioxazin) applied EPP or PRE controls most small-seeded broadleaf weeds and may suppress foxtail, common and giant ragweed, annual smartweed, Russian thistle and wild buckwheat. Valor does not control perennial weeds. Apply from 14 days prior to planting to just before soybean emergence.
Valor can be applied with glyphosate in early burndown programs in soybean. Valor requires a minimum of 0.25 inch of rain for activation and requires a bioassay prior to planting sensitive crops. Refer to the label for weeds controlled, rates and crop rotation restrictions.
Soybean Herbicide Injury/Symptomology
Acetanilide (Lasso, Dual, etc)
Leaf stunting, puckering and the “draw string” effect on the central vein or leaf midrib.
DNA (Trifluralin, Sonalan, Prowl)
Excessive rates with stress conditions may cause pruned roots and swollen or cracked hypocotyls.
Plant growth regulators
Leaf puckering, along with stem and branch twisting and epinasty. Leaf strapping, cupping and parallel veins may be prevalent at lower exposure rates
ALS inhibitors
Misapplication, drift or carryover of some ALS herbicides not registered on soybean may stunt soybean plants and cause yellow or chlorotic blotches on leaves. Labeled herbicides such as Raptor and Pursuit may intensify the symptoms of iron chlorosis. Tank-mixes of Harmony GT with Pursuit or Raptor are not recommended due to severe soybean stunting and leaf burn.
Contact – soil applied (Authority and Valor)
Authority: Some soybean varieties are susceptible to injury. See your seed dealer for a list. Symptoms are stunting and yellowing of soybean leaves. Valor may cause localized speckling from a “splash effect” after a rain storm. Speckling may occur only on bare soil where no crop residue exists.
Contact – POST (Aim, Blazer, Cobra, Flexstar)
Aim, Blazer and Flexstar may show localized speckling of soybean leaves. More serious injury may result if Aim is applied in wet or dewy conditions. Injury from Cobra may vary from speckling to severe leaf burn. New soybean growth after contact herbicide application is unaffected.
Contact – POST (Basagran)
Yellow chlorotic mottling in small patches on leaves. Areas of leaf burn may occur under stress conditions or hot temperatures. Injury is cosmetic and new growth is unaffected.
Triazine
Symptoms of atrazine carryover from high rates and high soil pH may be visible as leaf burn and desiccation from the bottom leaves progressing up the plant and from leaf tips inward. Symptoms from metribuzin may be similar to atrazine where high rates are used.
Glyphosate (conventional soybean)
Symptoms from drift are expressed early on new growth as stunting and leaf yellowing. Symptoms will progress to older plant tissue. Plants may remain stunted and affected plant tissue may die seven to 14 days after exposure, depending on the herbicide concentration and growing conditions.
Insect Management in Soybean
Janet J. Knodel, Extension Entomologist
Producers should scout soybean fields on a regular basis to minimize insect pest damage and adopt integrated pest management (IPM) strategies, such as the use of economic thresholds and combining various control methods when available.
Prior to the first detection of soybean aphid in the U.S. in 2000, soybean grown in the north-central U.S. rarely was damaged by insects; indeed, it was considered to be a low-risk crop when grown in rotation with corn or wheat. Since soybean aphid has become a chronic major insect pest of soybean, inputs for control of insect pests have increased dramatically.
Other insect pests that occasionally infest soybean include spider mites, bean leaf beetles, seedcorn maggots, potato leafhoppers, cutworms, armyworms, various foliage-feeding caterpillars and grasshoppers. Significant progress with soybean pest management has been made and will continue into the future to aid successful soybean production.
A growing-season calendar shows the major soybean insect pest problems and the time of occurrence in the northern production area of North Dakota and Minnesota (Figure 5).
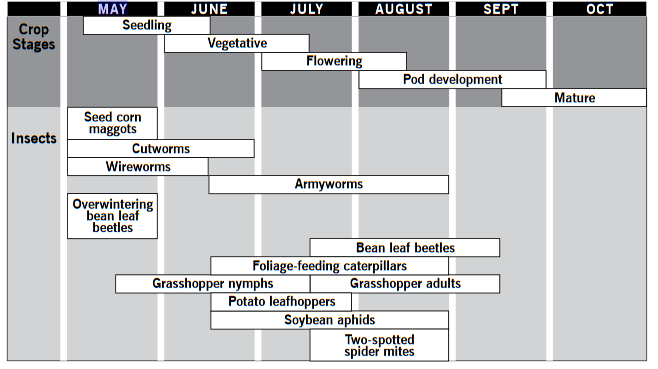
Figure 5. A growing-season calendar indicating the time of occurrence of soybean insect pests.
For insecticides registered in soybean, please consult the soybean section of the latest version of the NDSU Extension “North Dakota Field Crop Insect Management Guide”.
Estimating Defoliation Damage
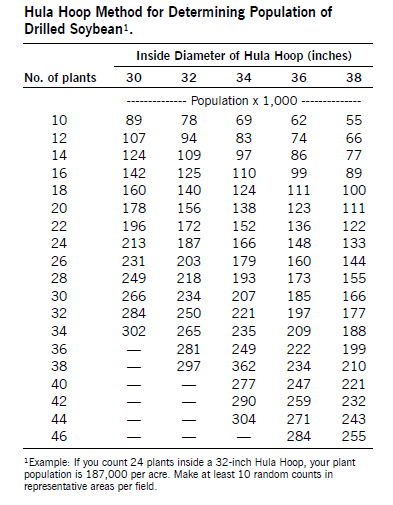
In soybean, field scouting to assess insect populations is based on the number of insects per foot of row, insects per plant, sweep net sampling or the level of defoliation.
Insects per foot of row are determined by shaking plants over the inter-row space, on which a strip of cloth has been laid. Count the total number of insect pests per foot of row that fall on the cloth.
If sampling narrow-row or drilled soybean, the use of a “Texas vertical beat sheet” should be considered. The vertical beat sheet is made from a piece of galvanized metal flashing or similar stiff material 36 inches wide and 32 inches tall that is crimped at the bottom to form a collecting trough 4 inches wide. Place the device next to the row and shake the plants against the vertical surface. Insects dislodged from plants collect in the trough, where they can be counted or collected.
The percent of defoliation is determined by estimating the amount of leaf tissue loss based on visual inspection of randomly selected plants. Examples provided (Figure 6) are guidelines for estimating loss for individual leaflets. Actual defoliation estimates made for pest management decisions are based on estimated leaf area lost from the entire plant.

Figure 6. Soybean defoliation levels.
The growth stage of the soybean plant is important when making pest management decisions. Under most conditions, moderate defoliation early in the season has little effect on final bean yield. As plants reach the flowering and pod-filling stages, defoliation poses a greater threat to yield. For example, research indicates that the soybean plant can sustain a 35 percent leaf loss prior to the pre-bloom period. From pod-set to maturity, the plant can tolerate only a 20 percent defoliation level.
Armyworms [Lepidoptera: Noctuidae: Pseudaletia unipuncta (Haworth)]
Armyworms are greenish-brown with longitudinal stripes. Full-grown larvae are smooth, striped and almost hairless. Armyworms feed for three to four weeks. When full grown, larvae are 1½ to 2 inches long.
Armyworm larvae have six growth stages, or instars. The final instar lasts about 10 days, and they consume large amounts of plant material during that time.
Armyworms are inactive during the day, resting under plant trash, and clumps of grass or lodged plants. They feed at night or on cloudy days, crawling up on plants and consuming foliage. Due to their habit of feeding at night, armyworms may go undetected until significant damage has occurred.
Armyworms do not overwinter in the region. Moths migrate from southern states in late spring and early summer. This helps explain the sporadic infestations that occur. When moths arrive, they prefer to lay their eggs in moist, shady areas, usually where grasses have lodged. Infestations that develop within soybean fields are often due to grassy weed problems.
Armyworms are more of a problem in small grains and corn. Damage to soybean can occur when the armyworms’ usual host plants become exhausted due to feeding or dry conditions. When their food is depleted in the hatching site, the armyworms may move in large numbers or “armies,” eating and destroying plants or crops in their path.
Threshold
Control of armyworms is recommended when 25 to 30 percent of the foliage is destroyed or if significant injury to pods is evident. Most often in soybean, infestations are due to migrating armyworms. Under these circumstances, treatment of a couple of swaths ahead of the migrating armyworms to establish a barrier strip and prevent further migration and injury may be all that is needed.
Bean Leaf Beetle [Coleoptera: Chrysomelidae: Cerotoma trifurcata (Förster)]
This beetle can vary from yellow to reddish brown and may have three to four black spots with a black border on the wing covers. Adults emerge from overwintering and move into bean fields as the seedlings emerge. The white larva develops in the soil, feeding on the roots and nodules. New adults emerging from mid-July to August feed on foliage and pods.
Feeding injury to leaves appears as small round holes between the leaf veins. Late-season feeding on the foliage and pods by the new adults that emerge in August can be more important than early season feeding, especially if viruses are present. This may increase the risk of virus transmission and cause secondary fungal and bacterial infections (rotting and discoloration). Bean leaf beetles are the vector of bean pod mottle virus.
Threshold
Due to a low incidence of this insect in North Dakota, no local control guidelines have been developed. Based on information from other regions where these insects are a common pest, a sweep net is used to determine if bean leaf beetles are present. Treatment would be recommended when three to seven beetles per sweep are found. Treatment thresholds based on defoliation are 50 percent defoliation during early vegetative, 40 percent defoliation during pre-bloom, 35 percent defoliation during bloom and 20 to 25 percent defoliation during pod set to fill.
Cutworms (Lepidoptera: Noctuidae)
Several cutworm species affect regional crops. The dingy cutworm, Feltia jaculifera (Guenée), overwinters as a partially grown larva and is one of the first cutworm species to cause problems during crop emergence from early to mid-May. The moth of the dingy cutworm is known to lay her eggs on sunflower heads from mid-July through September. Soybean and other crops following sunflower in rotation are at greatest risk of injury by this cutworm.
Other cutworms – the redbacked, Euxoa ochrogaster (Guenée) and the darksided, Euxoa messoria (Harris) – overwinter as eggs, which hatch in mid to late May. Eggs are laid in the fall and survive in weedy, wet and reduced-tillage areas. Feeding injury by these cutworms normally occurs in late May to mid-June.
Most damage by cutworms occurs when soybean plants are in the early stage of development. Damage consists of young plants being chewed off slightly below or at ground level. Some cutworm feeding injury may occur on foliage. Cutworms feed primarily at night. When checking soybean fields for cutworms during the day, dig down into the soil an inch or two around recently damaged plants; there you can find the gray to brown larvae.
Threshold
Economic thresholds for cutworm treatment decisions are not well-established. Treatment guidelines used through the years include when one cutworm (larva) or more is found per 3 feet of row and larvae are small (less than ¾ inch long). Another guideline is when 20 percent of plants are cut or when gaps of 1 foot or more exist in the plant row. When making a final decision, consider that surviving soybean is able to compensate for early stand reductions because of the plant’s long growth period and branching ability.
Foliage-feeding Caterpillars (Lepidoptera)
• Green Cloverworm, Cabbage Looper, Velvetbean Caterpillar, Thistle Caterpillar and Alfalfa Webworm: Populations of these foliage-feeding caterpillars (larvae) are considered occasional insect pests in North Dakota and little treatment to control them has been required. To sample for larvae, use a drop cloth or vertical beat sheet placed between two rows of plants. Dislodge the larvae from the plants and count them on the cloth or collection tray to arrive at an estimate of the number per row feet. Treatments also can be based on the average defoliation (see Estimating Defoliation Damage section near the beginning of Insect chapter).
• Green cloverworm [Erebidae: Hypena scabra (Fabricius)]: These caterpillars are green with two narrow white stripes down the side. When mature, the worms are 1¼ inches long. These caterpillars have only three pairs of fleshy prolegs on the abdomen, plus the pair on the back tip. When moving, the worms arch the middle of the body, or “loop.” Young worms scrape leaf tissue, creating a transparent skin, or “window,” on the leaf surface. Older clover worms eat holes in the leaves.
• Cabbage looper [Noctuidae: Trichoplusia ni (Hübner)]: These caterpillars are light to dark green with lighter-colored stripes along the side and on the top, running the length of the body. When mature, the larva is 1½ inches long. These caterpillars have only two pairs of fleshy prolegs on the abdomen, plus the pair on the back tip. When moving, the caterpillars also arch the middle of the body, or “loop.” These worms feed on leaves on the interior and lower portion of the plant. As defoliation occurs, worms feed higher in the plant. Feeding injury is similar to what the cloverworm causes.
• Velvetbean caterpillar (Noctuidae: Anticarsia gemmatalis Hübner): This insect does not overwinter in the region. Instead, moths migrate from southern locations. These caterpillars have dark lines bordered by lighter-colored, narrower lines running the length of the body. The background color ranges from a pale yellow-green to brown or black. These larvae have four pairs of fleshy prolegs to distinguish them from the cloverworm and the looper. Young velvetbean caterpillars feed on the underside of leaves in the upper portion of the plant. Older larvae consume the entire leaf, except for the leaf veins.
• Thistle caterpillar [Nymphalidae: Vanessa cardui (Linnaeus)]: This insect is the larva of the butterfly known as the painted lady. This butterfly does not overwinter in the region but migrates from southern locations each spring. These caterpillars are brown to black, with yellow stripes along each side of the body. They are covered with spiny scoli (fleshy structures) that give the caterpillar a prickly appearance. Full-grown larvae are about 1½ inches long. The caterpillars feed on the leaves, webbing them together at the feeding site.
• Alfalfa webworm [Crambidae: Loxostege cereralis (Zeller)]: These larvae are 1 inch long when fully grown. They are greenish to nearly black, with a light stripe that runs down the middle of the back. They have three dark spots, each with hairs, on the side of each segment. These larvae feed for about three weeks. Infestations are characterized by light webbing over the leaves. Beneath the web is where the larvae feed, consuming the leaves. These larvae move very rapidly, forward or backward, when disturbed.
Threshold
Control of these foliage-feeding caterpillars normally is not warranted until greater than 30 percent of the foliage is destroyed prior to bloom, or when 20 percent of the foliage is destroyed after bloom, pod set or fill has been reached. This usually requires an average infestation of four to eight larvae per row foot.
Grasshoppers (Orthoptera: Acrididae)
In the northern Great Plains, grasshopper egg hatch normally begins in late April to early May. Most grasshoppers emerge from eggs deposited in uncultivated ground. In spring, soybean growers should expect to find grasshoppers feeding first along bean field margins adjacent to noncrop sites, where nymphs are hatching. Later infestations may develop when grasshopper adults migrate from harvested small-grain fields. Grasshoppers will feed on leaves and pods, chewing holes in them. Soybean fields often become sites for significant egg laying from migrating grasshopper adults.
Threshold
The threatening rating is considered the action threshold for grasshoppers. For example, grasshopper control is advised whenever 50 or more small nymphs per square yard can be found in adjacent, noncrop areas, or when 30 or more nymphs per square yard can be found in the field. When 20 or more adults per square yard are found in field margins or eight to 14 adults per square yard are occurring in the crop, treatment would be justified. Because estimating the number of grasshoppers per square yard is difficult when population densities are high, pest managers can count grasshoppers collected from four 180-degree sweeps when using a 15-inch sweep net and use that value as an estimate for the number of adult (or nymph) grasshoppers per square yard.
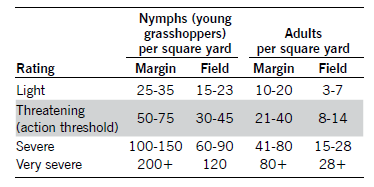
Many of the grasshopper infestations in soybean will be heaviest on field margins. Treating these areas early may lessen the total number of grasshoppers successfully entering a field.
Soybean is most sensitive to defoliation during pod development (growth stages R4 to R6). During this time, plants can tolerate up to only a 20 percent defoliation.
Of greater concern is direct feeding damage to pods and seeds. Grasshoppers are able to chew directly through the pod walls and damage seed. If grasshoppers injure more than 5 to 10 percent of the pods, an insecticide application is recommended.
Potato Leafhopper [Hemiptera: Cicadellidae: Empoasca fabae (Harris)]
The adult is about 1/8 inch long, pale green and wedge-shaped. Adults are very active, jumping or flying when disturbed. Nymphs are wingless. Adults and nymphs run backward or sideways rapidly when disturbed.
Nymphs feed on the underside of the leaf, usually completing their growth on the leaves near where they hatched. Large numbers of adults may appear early in the season, but their presence is dependent on migration from the southeastern U.S.
Soybean with moderate to dense pubescence, or plant hairs, are tolerant to leafhopper infestations. The short plant hairs form a barrier that discourages leafhoppers from feeding and laying eggs on plant tissue. When feeding does occur, damage by leafhoppers is referred to as “hopper burn.” Foliage becomes dwarfed, crinkled and curled. Small triangular brown areas appear at the tips of leaves, gradually spreading around the entire leaf margin. Potential damage to soybean by potato leafhopper is not fully understood. Damage is more likely when drier growing conditions occur.
Threshold
The threshold for spray decisions is an average of five leafhoppers per plant in the vegetative stages, and nine leafhoppers per plant in early bloom stages.
Soybean Aphid [Hemiptera: Aphididae: Aphis glycines (Matsumura)]
Soybean aphid was first detected in the U.S. in 2000 and has spread through soybean production areas in the north-central U.S., including North Dakota in 2001. Since its introduction, soybean aphid has become a major insect pest of soybean throughout the Midwest.
Foliar insecticides are the primary management tactic for aphid control. However, multiple years of research have shown that natural enemies, including predators (lady beetles) and parasitic wasps, often can keep soybean aphids below the economic threshold in non-outbreak years. Another nonchemical management tactic that shows promise for controlling soybean aphid is the use of genetically based aphid-resistant soybean varieties.
Soybean aphid is small, about 1/8 inch, lime green with black cornicles (“tail-pipes”) and a pale-colored cauda (tail projection). Nymphs (or young) are smaller yet.
Aphids have piercing-sucking mouthparts and feed on plant sap. When infestations are large, infested leaves are wilted or curled. Aphids excrete honeydew, a sweet substance that accumulates on surfaces of lower leaves and promotes the growth of sooty mold.
Soybean aphids colonize tender leaves and branches from early vegetative through reproductive plant stages. Later, as vegetative plant growth slows, the aphids slow their reproductive rate, move down to the middle and lower part of the plant, and feed on the undersides of leaves. Toward the end of the season, the colonies again begin to increase in number rapidly. These increases are followed by a migration to the overwintering host, buckthorn. Soybean aphids overwinter as eggs near the buds of buckthorn.
Threshold
The guidelines for making soybean aphid treatment decisions are:
• Begin scouting soybean fields at the V3 to V4 stage to determine if soybean aphids are present in fields. No treatment is recommended at this time and is discouraged so that insecticides do not reduce the presence of predators and parasites.
• The critical growth stages for making most soybean aphid treatment decisions are the late-vegetative to early reproductive stages (Vn to R3). Assessing aphid populations at these times is critical.
• The economic threshold from R1 (first flower) through R5 (beginning seed) is 250 aphids/plant and when populations are increasing actively in 80 percent of the field. At R6 (full seed), no insecticide treatment is recommended. Research trials throughout the north-central states have not demonstrated a yield benefit for soybean aphid management at the R6 and later stages.
Soybean Aphid Resistant to Pyrethroid Insecticide
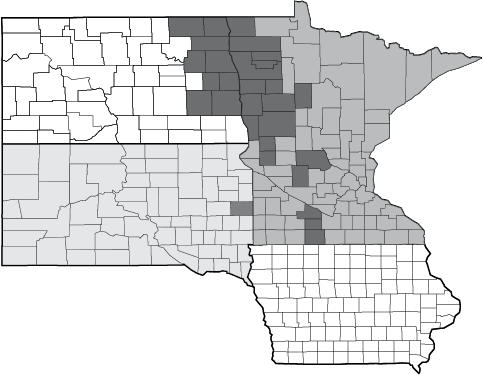
Pyrethroid resistance first was documented in North Dakota in 2017. In the map, dark gray counties show where pyrethroid performance issues occurred for soybean aphid management in 2017. A total of nine counties were reported in eastern North Dakota (Figure 7).
Koch et al.
U of MN, NDSU, SDSU, ISU
Map by B. Potter
Figure 7. Field reports (counties indicated in ) of pyrethroid failure for control of soybean aphid in 2017.
Soybean aphids were collected from six soybean fields with reported pyrethroid failure, and these aphids were evaluated for resistance to two pyrethroid insecticides (bifenthrin and lambda-cyhalothrin) using the glass vial bioassay. Aphid populations from Casselton, Hope, Emerado, Lodema and Osnabrock had significantly less mortality than the laboratory population for bifenthrin, and less mortality in Grafton (two fields), Lodema and Osnabrock for lambda-cyhalothrin. These North Dakota soybean aphid populations are considered resistant to the pyrethroid insecticides.
Due to low populations of soybean aphids in 2018, testing for pyrethroid resistance was difficult. However, we were able to test one site that had soybean aphid populations that were resistant to bifenthrin only in 2017. After rearing the aphids in the lab to get enough numbers to run the glass vial bioassay, these soybean aphids ended up being “susceptible” to bifenthrin and lambda-cyhalothrin. All soybean aphids were dead at the four-hour mortality assessment.
This suggests that the source of our pyrethroid-resistant soybean aphids in North Dakota (2017) may be aphids that migrated into the state from neighboring states, and resistant populations may not be established or overwintering in North Dakota. However, we need more research data to validate this hypothesis.
To reduce development of insecticide resistance in soybean aphids, Extension entomologists recommend:
• Scout fields regularly.
• Use the economic threshold to aid in decision-making, prevent unnecessary insecticide applications and conserve natural enemies.
• Rotate the mode of action (or insecticide class) if more than one applications is necessary in a season.
• Do not use the same mode of action (or insecticide class) repeatedly year after year.
• Avoid using the lowest rate of insecticide on the label. Use high rates.
• Do not use premix insecticides containing two insecticides of the same or two different modes of action because premixes have lower amounts of active ingredient per insecticide.
For more information, please consult the NDSU Extension publication “Management of Insecticide-resistant Soybean Aphids”.
Seedcorn Maggot [Diptera: Anthomyiidae: Delia platura (Meigen)]
Seedcorn maggots attack soybean seed, preventing sprouting or weakening the seedlings. The yellowish-white maggot burrows into the seed, emerging stem or the cotyledon leaves. Damage to the seedlings results in a condition called “snakeheads,” or plants without cotyledon leaves.
Adult flies emerge in the spring when soil temperatures reach 50 F. They deposit eggs in soil with abundant organic matter and decaying crop residue, or on the seed or seedling. Injury from seedcorn maggots is usually most severe during wet, cold springs and in fields with high organic matter soils. When cool, wet conditions occur during planting, the slow germination and emergence of the seedling extends the period of time it is vulnerable to the maggot’s feeding.
Threshold
When conditions are wet and cool, or when planting into high crop residue conditions, insecticide seed treatments provide the best defense against damage.
Two-spotted Spider Mites (Acari: Tetranychidae: Tetranychus urticae Koch)
Adult spider mites are small (less than 0.2 inch) and greenish white to orange red, and have two dorsal spots and four pairs of legs. Nymphs are smaller than adults and have three to four pairs of legs. Magnification is necessary to see mites.
Host plants for spider mites include soybean, dry beans, alfalfa, corn, vegetables, ornamentals and trees. Mites overwinter as eggs on vegetation. The life cycle of spider mites can be completed in only five to 14 days, with the fastest development rates occurring above 91 F.
Each female lives for 30 days and produces about 300 eggs during her lifetime. In hot, dry weather, natural fungal diseases of mites are slowed and populations can increase from a few individuals to millions within a few generations. Mites thrive on the stressed plants that are nutrient-rich.
Leaf injury symptoms appear as stippling first and then progress to yellowing, browning or bronzing as feeding injury increases, and eventually leaf drop. Feeding injury causes water loss from the plant and reduces the photosynthetic ability of the plant. In severe cases, premature leaf senescence and pod shattering, and even plant death can occur.
When severe mite infestations occur during late vegetative and early reproductive growth, a 40 to 60 percent yield loss between treated and untreated soybean has been demonstrated in other north-central states. Be sure to scout during full pod (R4) through beginning seed (R5) stages because these crop stages are the most important contributors to soybean yield. Spider mites can cause yield reduction as long as green pods are present.
When scouting for spider mites, look on the underside of leaves and lower foliage at the field edges first for tiny mites and fine spiderlike webbing. A quick sampling procedure to determine whether mites are present is to hold a piece of white paper below leaves, then tap them to dislodge the mites. The mites appear as tiny dust specks; however, they will move slowly after being knocked off the leaf. Dislodged predatory mites will move faster than the two-spotted spider mite.
Another sampling measure involves pulling up plants and examining the underside of the leaves from the bottom of the plants upward. When spider mites need to move due to diminishing food supply, they climb to the top of plants and are dispersed by the wind through “ballooning,” so they can spread quickly within a field or to adjacent fields.
Infestations typically are first noted near field edges and fields near alfalfa (a preferred host). Products labeled for mite control often do not give adequate control and the population of mites may rebound quickly to pretreatment levels or higher. When rain and humidity are present, natural reductions in mite populations occur due to infection by a fungal pathogen.
Conditions that are good for the development of the pathogen are temperatures cooler than 85 F, with at least 90 percent relative humidity for 12 to 24 hours. Mites usually become a problem when hot, dry weather occurs. When a production area has low rainfall, the region can become a “hot spot” for mite injury and a source of mites migrating to neighboring areas.
Threshold
Deciding whether to treat is difficult. No specific threshold has been developed for two-spotted spider mite in soybean. Sample plants at least 100 feet into the field. Walk in a “U” pattern and sample two plants per location at 20 different locations. Assess mite damage using the following scale from the University of Minnesota:
0 - No spider mites or injury observed
1 - Minor stippling on lower leaves; no premature yellowing observed
2 - Stippling common on lower leaves; small areas or scattered plants with yellowing
3 - Heavy stippling on lower leaves, with some stippling progressing into middle canopy; mites present in the middle canopy, with scattered colonies in upper canopy; lower-leaf yellowing common; small areas with lower leaf loss may occur (spray threshold)
4 - Lower-leaf yellowing readily apparent; leaf drop common; stippling, webbing and mites common in the middle canopy; mites and minor stippling present in the upper canopy (economic loss)
5 - Lower leaf loss common; yellowing or browning moving up plant into the middle canopy; stippling and distortion of upper leaves common; mites present in high levels in the middle and lower canopy
If spider mites are above threshold when significant pod or seed fill remains, an organophosphate insecticide (for example, Lorsban, Dimethoate) is recommended vs. a pyrethroid insecticide. Pyrethroids (for example, Asana, Baythroid, Mustang Max, Warrior) tend to flare (increase) mite populations seven to 10 days after application. Reasons for an increase in mite populations include: disruption of the natural enemies that control spider mites (predatory mites); increased movement of mites out of fields and increased reproductive rates of female mites.
Early detection facilitates timely and effective rescue treatments. Current insecticides for soybean provide short-term protection, about seven days, from mites and do not kill mite eggs. Fields will need to be monitored continually for resurging populations.
The efficacy of an insecticide can be improved significantly with sufficient coverage (greater than 18 gallons per acre of water) and application at high pressure to penetrate foliage. Insecticides labeled for mite control have a 21-day (Dimethoate) to 30-day (Cobalt) harvest interval. Consequently, if infested fields still have green seeds but seeds are filling, accepting some yield loss from mites and not treating may be better than treating and being unable to harvest.
IPM for Soybean Aphids and Spider Mites
When scouting soybean fields, consider which insect pests (soybean aphid and spider mites) are present and their population levels. If the heat and drought stress continues, the risk for spider mites increases and the risk for soybean aphids is reduced (increased mortality and decreased reproductive rate due to hot temperatures greater than 90 F). If heavy rains occur, mite and aphid populations can collapse.
Mite infestations often are concentrated early in field edges, and spot treatment can be feasible and more economical. However, under dry conditions, mites usually will occur throughout the field, and spot treatments are not effective in controlling mites and unlikely to prevent the infestation from spreading. Early detection facilitates timely and effective rescue treatments.
Wireworms (Coleoptera: Elateridae)
Wireworms are most likely to be problems when soybean follows pasture or grassland. Infestations often are found in coarse-textured soils (sandy loam) where moisture is abundant, perhaps in low spots of fields.
Thresholds
You have no easy way to estimate wireworm infestations. Two methods are used:
• Soil Sampling: Sample 20, well-spaced, 1-square-foot sites to a depth of 4 to 6 inches for every 40 acres being planted. If an average of one wireworm per square foot is found, treatment would be justified.
• Solar Bait Trap: In September, establish bait trap stations for two to three weeks before freeze-up. Place bait stations randomly through the field, but represent all areas of the field. You should have 10 to 12 stations per 40-acre field. Place 1 cup of wheat and 1 cup of shelled corn in a 4- to 6-inch-deep hole. Cover grain with soil and then an 18-inch-square piece of clear plastic. Dig up the grain after two weeks in the field. If an average of one or more wireworm larvae is found per station, treatment would be justified.
Seed Treatment: Insecticide seed treatments should be applied as commercial or on-farm application for managing wireworms in soybean.
Disease Management and Identification
Sam Markell, NDSU Extension Pathologist
Dean Malvick, University of Minnesota Extension Pathologist
Berlin Nelson, NDSU Pathologist
Many important diseases of soybean in the North Dakota and northern Minnesota region may affect yield and quality of the crop. Accurate identification of the problem is the first step in managing these diseases. Once identified, specific management techniques to address the problem may benefit soybean growers.
The information in this chapter is designed to aid in identification, prevention and management of soybean diseases. Pictures can be found in the picture section of this publication. Below are general guidelines for managing soybean diseases.
• Use high-quality seed. Certified seed will minimize the introduction of soybean pathogens. Avoid using seed produced on fields with diseases that can be seed-borne.
• Use crop rotation. Soybean diseases, especially root and stem diseases and soybean cyst nematodes, often increase when soybean crops are grown in close rotations. Lengthening rotations to three or four years between soybean crops allows natural processes to reduce pathogen populations. Some crops such as dry bean and sugar beet may be infected by some pathogens that attack soybean. Have diseases accurately identified so sound decisions can be made on the use of rotation crops.
• Scout fields for disease. Record the incidence of disease; such information can be used to make good decisions on management practices.
• Strengthen the soybean plant. Use good cultivation practices to promote the growth of soybean plants. Provide adequate soil fertility, avoid soil compaction, enhance drainage, control weeds and avoid herbicide damage.
For more information:
• “Soybean Disease Diagnostic Series”: Pocket guide designed specifically to help growers identify soybean diseases in North Dakota and Minnesota, NDSU Extension publication PP1867.
• University of Minnesota Extension Soybean Disease website: Designed to provide fundamental information on identification and management of soybean diseases in Minnesota.
• North Dakota Pest Management App: Multi-function app containing information on labeled pesticides (updated annually), diagnostic images and interactive tools for many crops grown in our region, available for Android and Apple devices
• “North Dakota Field Crop Plant Disease Management Guide”: Booklet containing information on all labeled pesticides (updated annually) for crops grown in our region. NDSU Extension publication PP622.
• The SCN Coalition: Resource center for soybean cyst nematode management, including videos, downloadable materials, state-specific information, available at www.thescncoalition.com
• Soybean Research Information Initiative: Resource center for soybean disease information in the north-central U.S., maintained by the checkoff-funded North Central Soybean Research Program, available at www.soybeanresearchinfo.com
• Crop Protection Network: Resource center containing information on many diseases of several crops developed by university plant pathologists across the U.S. and Canada.
Below Ground
In general, the most significant diseases in our region, such as root rots and the soybean cyst nematode, are found underground.
Soybean Cyst Nematode
The soybean cyst nematode (SCN), Heterodera glycines, is the most important pathogen of soybean in the U.S. This nematode is a microscopic roundworm that infects soybean roots. Extensive losses occur throughout infested areas. Losses greater than 50 percent have been measured in infested fields in North Dakota.
Nematodes easily spread from field to field by equipment contaminated with infested soil, wind-blown soil, overland flooding or animals carrying it. In addition, SCN can be carried in soil “peds,” small clumps of soil sometimes found associated with soybean seed.
SCN has occurred in southern Minnesota since the 1980s. In 2003, SCN was confirmed in North Dakota (Richland County). SCN has been confirmed in many eastern North Dakota and northern Minnesota counties, and is expected to continue to spread.
Symptoms
Infection by SCN often will not result in obvious above-ground symptoms unless plants are stressed or the nematode densities are high. Above-ground symptoms include stunting and yellowing, poor stands or just unthrifty plants. The roots can appear dark and decayed, and they have few, if any, nitrogen fixing nodules.
A diagnostic characteristic is the presence of white to yellowish lemon shaped female nematode cysts (about 1 millimeter [mm] in diameter) on the roots. These can be observed if the plant is removed from the soil carefully with a shovel and the soil is gently washed or shaken off the roots.
The severity of the symptoms is directly related to the amount of SCN in the soil. Warm, dry growing seasons tend to increase severity of the above-ground symptoms, while cool, wet years tend to decrease severity. Losses from SCN are usually much greater during droughts. Severity also tends to be higher in sandy soils rather than heavy-textured soils, and in soils with a higher pH.
Management
Effective management of soybean cyst nematode is accomplished only by using multiple management tools.
Maintain Fields Free of SCN
In fields where SCN has not yet been confirmed, reducing the likelihood of introduction is critical. In areas where SCN has been confirmed, limiting its spread to new areas in the field or neighboring fields can reduce the chance of widespread yield loss.
Reduce the likelihood of introduction by cleaning any equipment that was used in an infested area. Reduce the potential spread of SCN from infested fields to adjacent clean fields through reduced-tillage operations and practices that limit wind erosion and movement of soil. Also, use good-quality seed free of soil “peds.”
Identify SCN
Management begins by confirming the presence of H. glycines in the field. Visual confirmation of SCN white females (lemon-shaped cysts) on soybean roots in the mid- to late growing season can serve as confirmation of SCN. However, these are very hard to identify without experience.
Take the Test – Beat the Best
The most effective method to identify SCN in fields is to soil-sample and test your fields for SCN. Soil sampling should concentrate on the top 6 to 8 inches of the soil and close to plant roots.
The most effective sampling strategy varies by situation. In fields where SCN has not been confirmed, sampling should concentrate on areas of the field where the nematode may have been introduced, such as field entrances, shelter belts and previously flooded areas. In fields where SCN sampling is being done to monitor egg levels, gridded sampling may be most effective. Sampling can be done in the fall or spring.
Growers can sample the soils in their fields and send the samples to public (for example, the University of Minnesota Southern Research and Outreach Center in Waseca, Minn., or the NDSU diagnostic laboratory in Fargo, N.D.) or private laboratories where egg counts can be determined. Soil test results are used to determine if a field is infested, if an extended rotation is necessary or if a resistant variety should be used.
If SCN is confirmed, active management should begin. Economic thresholds for SCN levels are minimally effective in this region. SCN reproduces quickly and to very high egg levels in this northern soybean-growing area; thus, once a field is infested, growing susceptible varieties may increase egg levels very quickly.
Crop Rotation
Crop rotation is an effective method to reduce egg densities in soil. Rotation to nonhost crops for three to four years may reduce nematode populations, but many factors affect the reduction in egg densities; thus, the results in each field will vary. Data from North Dakota indicates that in heavily infested fields following rotations to nonhost crops for four to five years, egg densities in some fields were still high.
Nonhost crops include corn, sugar beet, alfalfa, potato, small grains and sunflower. Dry edible bean, certain lupines and crambe are hosts of SCN. Consult a list of susceptible crops before growing specialty crops in SCN-infested fields. Weed control is important because SCN will reproduce on a wide range of weeds.
Resistant Varieties
Resistant varieties are an effective tool to manage SCN. The majority of varieties have the same source of genetic resistance (PI88788). Effectiveness of PI88788 in varieties will vary, with some varieties being more resistant than others.
For severe infestations of SCN, selecting a variety with robust resistance, if known, is important. Long-term rotation strategies that also include soybean varieties with an additional source of resistance (such as Peking) are likely to be most effective and help slow nematode adaptation to the genetic resistance.
Seed Treatments
Nematicide seed treatments for SCN became widely available in the late 2010s, and new products likely will be developed and labeled relatively frequently. At the time of this printing, more than a half-dozen nematicide seed treatments are available. They range from biologicals to resistance inducers, fungicides and true nematicides. Consequently, effectiveness among them likely varies and may be influenced by many in-field factors.
Phytophthora Root Rot
Phytophthora root rot is a major disease of soybean, especially in areas where soybean has been cultivated for many years. The disease is caused by the fungal-like pathogen Phytophthora sojae. Yield losses can be substantial, and entire fields have been destroyed.
The disease is common in the Red River Valley. The pathogen survives in soil as spores called oospores, which are produced in infected plants. When the soil water content is high, the spores germinate and infect the roots. Infection and disease development can occur at any stage of plant development, but most commonly are observed and cause damage at the seedling or young plant stage.
Disease is most common in heavy, compacted clay soils and fields subject to flooding. Flooding rains, especially near the time of planting, favor disease development. Reduced tillage, especially no till, is reported to increase damage.
The pathogen does not infect other crops grown in this region naturally. Only three Lupinis spp. and soybean are natural hosts. Many pathotypes (similar to races) of P. sojae overcome many resistance genes, and as more acreage is cropped to soybean and more resistance genes are deployed, we expect that the pathotype frequency will change.
Symptoms
Symptoms include seed rot and pre and postemergence damping off and wilting of young plants. These are common in flooded soils and often are misidentified as water damage. On older plants, leaves may become yellow and plants will wilt, with wilted leaves remaining on the plant. The lateral and tap roots are destroyed. A dark brown discoloration often appears on the lower portion of the stem. Disease is usually patchy in the field, often occurring in low or flooded areas.
Management
Planting resistant varieties is an important method to manage Phytophthora root rot. However, at the time of printing, no resistance gene can manage all the pathotypes of Pyhtophthora found in our region. We recommend that growers choose a resistance gene and then monitor the field for Phytophthora root rot. If the disease is significant in the field, select a variety with a different resistance gene the next time the field is planted.
Reducing saturated soil conditions – for instance utilizing subsurface (tile) drainage – is a method to reduce Phytophthora in the field.
Some varieties have tolerance and partial resistance to Phytophthora root rot. This type of resistance can be effective against all pathotypes. These varieties may not lose yield under low to moderate disease pressure but can be damaged severely under high disease pressure. Crop rotation is not an effective method to reduce disease because the oospores are long-lived in soil.
Fungicide seed treatments will help protect plants from infection for the first few weeks after planting. Metalaxyl and mefenoxan have efficacy against Phytophthora root rot and have been used for many years in our region. In the 2010s, the additional efficacious fungicide seed treatments ethaboxam and oxathiapiprolin became available. Ethaboxam and oxathiapiprolin have different modes of action than Metylaxyl and Mefenoxam. Rotation of these products will help prevent the development of fungicide resistance in our region.
Rhizoctonia Damping off and Root Rot
The fungus Rhizoctonia solani causes pre and postemergence damping off and root rot of young and adult plants. When soil populations of Rhizoctonia are high and soil is warm and moist for the first month after planting, pre and postemergence damping off can reduce stands by 50 percent or greater. Generally, Rhizoctonia on soybean is a seedling disease, but damage has been observed on older plants. The pathogen survives in the soil and is common in this region.
Symptoms
Seed decay and brown to reddish lesions on seedling stems and roots just below the soil line are symptoms. These lesions may girdle stems and kill the plant. On older plants, the pathogen causes a reddish-brown cortical root rot, which may extend into the base of the stem. Plants may appear unthrifty or may die. Root rot can greatly reduce nodulation.
Damage from Rhizoctonia solani commonly is observed in areas with a long history of soybean production with close rotations or during weather conditions not favorable for seed germination and rapid growth of seedlings. Various anastomosis groups (AG) of R. solani. AG 2-2, AG 4 and AG 5 are most common on soybean, but AG 3 occasionally is found.
AG 3, generally found on potato, is weakly pathogenic on soybean, but AG 2 2 can be highly pathogenic, especially at high temperatures. AG 4 and AG 2 2 are common on sugar beet.
Crop rotation practices may impact the amount of R. solani in the field, and therefore, affect the severity of the disease. Disease severity appears greater in plants showing iron deficiency chlorosis.
Management
Crop rotation to nonsusceptible hosts such as small grains will reduce populations of Rhizoctonia in the soil. Avoid close rotations with sugar beet if you have evidence of Rhizoctonia in the field. Close rotations with dry beans also may increase incidence of disease.
Some seed treatments and good seedbed preparation can reduce damping off. Cultivating soil to hill up around stems promotes lateral root growth and may lessen the effect of root rot on older plants.
Fusarium Root Rot
Fusarium root rot caused by Fusarium solani and other Fusarium species can cause damping off of seedlings and root rot on older plants. Infected seedlings can result in poor stands, late emergence or stunted plants.
Fusarium root rot often has been observed in association with stressed plants, such as in drought conditions or with herbicide damage. However, high populations of the pathogen in the soil may result in disease development under good growing conditions.
The pathogen may interact with other pathogens such as Rhizoctonia or the soybean cyst nematode to cause disease. Disease severity may be greater in plants showing iron deficiency chlorosis.
Symptoms
Infected seedling roots will show reddish or dark brown discoloration and decay. The disease at this stage may be misdiagnosed as Rhizoctonia because symptoms are similar. Symptoms on older plants consist of reddish-brown lesions on lateral roots and the tap root. In advanced stages of the disease, the cortex decays, the roots are black and fissures develop in the dead surface tissues of the tap root. Few nitrogen-fixing nodules may be on the roots.
Plants may appear stunted or unthrifty, and the leaves may be yellowing while the veins remain green for a short time. The leaves eventually become completely yellow, then die from the edges inward and fall from the petioles.
Management
Crop rotation will lower populations of the pathogen in the soil. When you see evidence of this disease, avoid dry beans in close rotations because the pathogen can infect dry beans.
Most varieties appear to be susceptible to Fusarium root rot. Fungicide seed treatments can reduce damping off by F. solani.
Damage to seedlings often occurs during weather conditions not conducive to rapid seed germination and plant emergence. Ridging soil around the base of the plants can promote root growth and reduce damage to root rot in older plants.
Use high-quality seed, plant in warm, well-drained soils, reduce soil compaction and provide good fertility.
Sudden Death Syndrome
Sudden death syndrome (SDS) is a severe disease of soybean that occurs in Minnesota and may spread north in the future. The disease is caused by Fusarium virguliforme. Yield losses from SDS can be severe when symptoms occur early during flowering.
Symptoms
SDS symptoms generally begin on the leaves at or just after pod-fill stages begin. Symptoms at first are scattered circular to irregular-shaped interveinal yellow spots that produce a mottled appearance to the leaves. The yellow tissue then dies and turns brown. The upper leaves are the first to defoliate; complete defoliation can occur when the disease is severe.
Flower and pod abortion can occur. Plants showing severe leaf symptoms also may have decay of roots. The disease usually first appears in patches in fields, which expand in subsequent years.
Disease development is associated with wet conditions early in the growth of soybean plants, and warm temperatures and heavy rainfalls during and after flowering. Foliar symptoms of SDS can be similar to those caused by brown stem rot.
Management
Selection of a soybean variety with reduced susceptibility to SDS may be prudent, if available. Fungicide seed treatments also may be available. If the soybean cyst nematode is present in fields with SDS, management of the nematode may help reduce SDS severity.
Dry edible beans are a host of the SDS pathogen; infected dry edible beans do not express the classic foliar SDS symptoms.
Because SDS is favored by high water content in the soil, practices that encourage drainage (including subsurface drainage) will help minimize disease development. Reducing soil compaction can reduce severity of SDS.
Above-ground Diseases
White Mold (Sclerotinia stem rot)
White mold of soybean is a common disease caused by the fungus Sclerotinia sclerotiorum. It can cause seed yield reductions, particularly when soybean is planted in infested soil, the plant canopy is dense and prolonged periods of wet weather (a major factor in disease development) occur. The disease rarely is observed when dry periods persist in July and August.
Besides seed yield reductions, the disease also results in reduced seed quality and seed contaminated with the black sclerotia of the fungus.
Sclerotinia overwinters as sclerotia in soil. The sclerotia germinate to form small mushrooms called apothecia that produce spores termed ascospores. The ascospores utilize senescing flower tissue as a food base and then infect the stems of the plant; the disease is tied closely to cool and wet weather during flowering.
Symptoms
Symptoms usually are observed after the canopy has closed. Dead plants are generally the first symptom observed. An inspection under the canopy will reveal a cottony, white mycelial (fungus threads) growth on stems, leaves or pods. Lesions develop on main stems and side branches.
Stems may appear bleached and sometimes shredded from advanced decay. Dark sclerotia form in and on diseased tissue. Seeds in diseased pods are usually shriveled and may be infected by the fungus or replaced by sclerotia. When a field with white mold is harvested, the seed almost always is contaminated with sclerotia.
Yield losses usually occur when incidence of disease is 15 percent or greater. In North Dakota, estimated yield loss per 10 percent disease incidence ranges from 1 to 3.4 bushels per acre.
The pathogen has an extensive host range of more than 370 plant species and causes diseases on a crops such as sunflower, dry bean, canola, alfalfa, buckwheat, lupine, mustard, potato, Jerusalem artichoke, safflower, lentil, flax, field pea and many vegetables. Many common broadleaf weeds such as marsh elder, lambsquarters, pigweed, Canada thistle and wild mustard also are hosts.
Management
Many management tools are available for white mold, including crop rotation, planting less susceptible varieties, avoiding planting on soils heavily infested with Sclerotinia, reducing the planting rate, wider row spacing and timely fungicide application. However, when the environment highly favors white mold development (cool temperatures and frequent, prolonged rains) the disease still may cause problems even if multiple tools are used.
Soybean fields should be monitored for disease incidence. Check the seed hopper at harvest for the presence of sclerotia. As the disease begins to increase in a field, the rotation time to nonsusceptible crops such as small grains and corn should be increased.
Crop rotation will reduce populations of sclerotia in soil but will not entirely eliminate the pathogen. We do not recommend that you plant susceptible crops such as dry edible bean and sunflower during the rotation. If you rent land, find out the disease and cropping history before making planting decisions.
Although common soybean varieties adapted for this region are susceptible to white mold, some varieties are less susceptible than others. Information on variety susceptibility may be available from NDSU Extension and seed companies.
Do not use seed from a white mold-infected crop. Seed quality could be low, and sclerotia may be introduced into the field along with the seed. Also, maintain good control of broadleaf weeds because they can be hosts of Sclerotinia and can make the microclimate more favorable for white mold. When growing a susceptible crop under irrigation, avoid practices that favor a dense canopy and free water on the plant during flowering because these will create ideal conditions for disease development.
Many fungicides are labeled for management of white mold on soybean and are more are likely to be available in the future. In favorable environments, fungicides may reduce disease. Efficacy varies, so consulting fungicide trial data before selecting a fungicide is important. The pathogen commonly uses flowers to begin the infection process; thus, applications commonly are made shortly after the onset of bloom (R1) when rows are filling.
Septoria Brown Spot
Septoria brown spot, caused by Septoria glycines, is a common leaf disease that may develop throughout the season. How frequently economic loss occurs in North Dakota and Minnesota is unclear, but in most of the Midwest, little evidence is available to suggest that Septoria brown spot causes yield loss, except under the most extreme circumstances.
Symptoms
The disease first appears as pinpoint brown spots on the leaves on the lower parts of the plants. These spots may remain small or enlarge up to 3/16 inch, becoming irregular and angular and reddish brown to dark brown with age.
Severely diseased leaves turn yellow and fall off, with defoliation beginning on the lower leaves and progressing up the plant. Brown, irregularly shaped spots may develop on the stems, petioles and pods.
Septoria brown spot development is favored by warm, humid weather. Hot, dry conditions will arrest disease development, but it may resume if conditions improve. Rainy weather is especially favorable because Septoria spreads by splash dispersed spores. Disease development also is favored in areas with poor drainage. The brown spot fungus survives on soybean crop refuse and may be seed-borne.
Management
Active management of this disease in most years is unnecessary, and management techniques utilized for other diseases (for example, crop rotation) likely are sufficient. Fungicides are available, but disease pressure warranting their use in our area is unlikely.
Additionally, bacterial blight is much more common than brown spot (especially in the early part of the season) and the symptoms of both pathogens can be confused. Correct identification of the pathogen is essential.
Bacterial Blight
Bacterial blight is a common foliar disease in North Dakota and Minnesota, but it is unlikely to cause significant yield loss. Bacterial blight (caused by Pseudomonas syringae pv. glycinea) favors cool, humid weather.
The bacteria blight pathogen can be seed-borne and can survive on soybean crop residue. Bacteria readily enter wounds in the leaf, and rapid spread may occur following late-spring or early summer rain storms, hail or cultivation when the plants are wet.
Symptoms
The disease begins as small, greasy green, angular, water soaked spots; later, they turn yellow and then reddish brown. The spots are surrounded by a narrow yellow border. As the spots coalesce, portions of the leaf tissue fall out, and the leaves become torn and ragged.
Infected young leaves may be distorted and stunted. Severely diseased leaves may drop off. Occasionally, large black spots may develop on stems, petioles and pods. Seeds in infected pods may become slimy. Hot, dry weather will stop the development of bacterial blight.
Management
Do not use seed from a diseased field. Use crop rotation and bury soybean crop residue with tillage. Do not cultivate when the plants are wet. Some varieties are less susceptible. Because bacterial blight is caused by a bacterial pathogen, fungicides are not useful for control.
Downy Mildew
Downy mildew, caused by Peronospora manshurica, develops primarily in years with extended periods of cool, humid weather. Yield loss rarely is reported, but very localized outbreaks in the far northern counties were thought to have reduced yield by up to 5 to 10 percent in some fields in the 2010s.
Symptoms
Symptoms include yellow green to yellow spots on the upper leaf surface and a purplish or grayish downy fungal growth on the lower leaf surface, opposite the yellow green patches on the upper leaf surface. The yellow spots turn brown later in the season.
Pod infection may result in seeds that are dull white, cracked or covered with a white crust of overwintering oospores. If these white or encrusted seeds are planted, a small percentage of the emerging seedlings may be infected systemically with the downy mildew fungus, resulting in stunted plants. Leaves of systemically infected plants will have areas of green yellow tissues along the main veins and the leaf edges will be curled downward.
Management
Use crop rotation and bury infected crop residue by tillage. Use a seed treatment if planting seed from an infected field or seed that has a white crust on it. Few fungicides are labeled for downy mildew and little efficacy data exists.
Pod and Stem Blight
This disease, caused by Diaporthe fungal spp., is common in southern and central Minnesota but somewhat less so in North Dakota and northwestern Minnesota.
Symptoms
Symptoms include rows of raised black fruiting bodies that develop on the stem, and a random pattern of raised fruiting bodies that develop on the pods. Infected stems often are killed. Infected seeds are shriveled and cracked and may be covered with white fungal growth.
The pod and stem blight fungus survives on infected soybean crop refuse and can be seed-borne. It favors wet weather and crop injury as the crop nears maturity. If infected seeds are planted, plants may die on emergence.
Management
Use crop rotation. Use tillage to bury infected soybean crop residues. Plant high-quality seed that is nearly free of the pod and stem blight pathogen or use a seed treatment. Harvest promptly at maturity. Maintain adequate potash levels.
Stem Canker
Stem canker is relatively common in southern Minnesota and in South Dakota, but how frequently it occurs in North Dakota and northwestern Minnesota is unclear. Stem canker is caused by two different but related pathogens, each causing a distinct disease: Diaporthe caulivora (northern stem canker) and Diaporthe aspalathi (southern stem canker).
Symptoms
Early symptoms of stem canker are reddish-brown lesions that appear at the base of the leaf petiole or branches. Lesions may develop into sunken dark brown cankers with small black raised structures on the surface (perithecia). Plant parts above the lesion may die. Stem canker favors wet weather, and symptoms may be most likely observed beginning in mid-July following wet springs.
Management
Resistant varieties, tillage, foliar fungicides and crop rotation may be useful to mitigate stem canker where it has been a problem.
Brown Stem Rot
Brown stem rot (BSR) occurs in North Dakota and Minnesota and is capable of causing yield loss in our region. BSR is more likely to be severe in the presence of soybean cyst nematodes. Infection occurs through the roots and develops slowly until pods are filling.
Symptoms
Symptoms usually do not appear until late in the season. The most reliable symptoms develop inside the lower stem. When the stem is split open with a knife, the pith (central tissues) is brown. The internal browning may extend several inches or more above the soil line.
Leaf symptoms, which develop sporadically, consist of a yellowing followed by browning of tissues between the main veins. The veins remain green. Foliar symptoms can be similar to those caused by sudden death syndrome.
The best time to assess for brown stem rot is the R5 to R6 stage, when seeds are beginning to develop in pods at the four uppermost nodes. Any time that a field suddenly turns brown late in the season, rather than yellow green, the lower stems should be split and examined for brown stem rot.
The brown stem rot fungus survives several years in soybean crop residue. The disease develops during cool or moderate temperatures. The greatest damage occurs when cool and wet weather occurs during the early reproductive stage and is followed by hot and dry weather.
Management
At the time of this printing, resistant varieties with suitable maturity for North Dakota and northern Minnesota are unavailable. Use crop rotation, planting nonhost crops for three years. Small grains and corn are not hosts. Burying soybean crop residue to hasten its decomposition may reduce brown stem rot.
Charcoal Rot
Charcoal rot also occurs in North Dakota and northwestern Minnesota. Charcoal rot is caused by the fungal pathogen Macrophomina phaseolina. The pathogen has a very large host range, which includes corn, dry edible bean and sunflower.
Yield losses are most likely when plants are under water stress midway to late in the season. Hence, drought, high temperatures and sandy soils favor charcoal rot.
In 2017 and 2018, severe outbreaks of charcoal rot occurred in localized areas of eastern North Dakota. Yield losses to the most severely infected fields in 2018 likely exceeded 50 percent.
Symptoms
Symptoms include a light gray to silver discoloration at the base of the stem. If the epidermis is scraped off at the base of the stem, charcoal-colored microsclerotia are visible. Microsclerotia are very small, and may appear as tiny black specs or as if the stem were covered in charcoal dust. The pathogen survives as microsclerotia.
Charcoal rot often is observed first as a general loss of vigor in small areas in a soybean field. Plants in those areas may begin yellowing and appear to be maturing up to three or four weeks ahead of green areas in the field. Plants surrounding those areas begin pre-maturely senesce soon after, giving the misleading appearance of the disease “spreading.”
Management
Crop rotation and planting nonhost crops are important management tools. If irrigating, keep soil water content reasonably high at the end of the season. Encouragement of early canopy closure by increasing planting density may reduce the risk of charcoal rot, but it may increase risk of other diseases.
Virus Diseases
Virus diseases have not been a serious problem in this area, but in soybean-producing areas to the south, viruses have become a problem in recent years. The recent introduction of the soybean aphid into this area may result in virus problems because aphids are virus victors.
The two most common viruses that have been found in North Dakota and Minnesota are soybean mosaic virus (SMV) and bean pod mottle virus (BPMV). Numerous other viruses are known in soybean but not yet detected in this northern growing area.
The identification of a virus disease requires special techniques. Identifying a virus based on symptoms is very difficult.
Virus symptoms vary greatly but may consist of stunting, fewer pods, leaf mosaic (light and dark green areas), puckering, blistering, distortion, chlorosis or necrosis. Plants can be infected without showing symptoms. Seed mottling can occur, which is detrimental to the quality of food beans.
The severity of disease and the effect on yield is greatly affected by the plant stage at infection, the environmental conditions and the susceptibility of the variety. Yield losses can be substantial under heavy disease pressure. Seed can transmit SMV and the virus is vectored by aphids (Aphis glycines). The vector of BPMV is the bean leaf beetle (Cerotoma trifurcata).
Hail Damage
Hans Kandel and Greg Endres, Extension Agronomists
A hailstorm can cause yield losses in soybean ranging from slight to total destruction of the crop. Extensive research has been conducted to accurately predict the effects of hail damage on soybean yields. Results from these studies are used by hail insurance companies to assess yield losses and consequent adjustment payment made to clients.
Yield loss predictions are based on two factors: a) the growth stage at the time of damage and b) the degree of plant damage. Plant damage is classified as leaf defoliation, stand reduction, stem damage and pod damage.
Stand reduction is a measure of the number of plants killed by the storm. The pre-storm plant population is compared with the remaining stand seven to 10 days after the storm to determine the yield loss due to stand reduction.
To determine the pre-storm population, count the original number of plants (live plants and remnants of plants) in 10 feet of row. Repeat this step several times throughout the field to get a representative sample. Now convert the average stand per 10 feet of row to plants per acre, using the following formula:
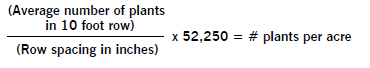
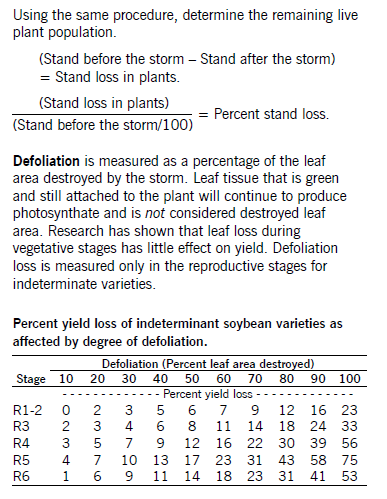
These tables are intended only to provide general guidelines to soybean yield losses due to hail injury. The percentage of nodes cut off or broken were not included.
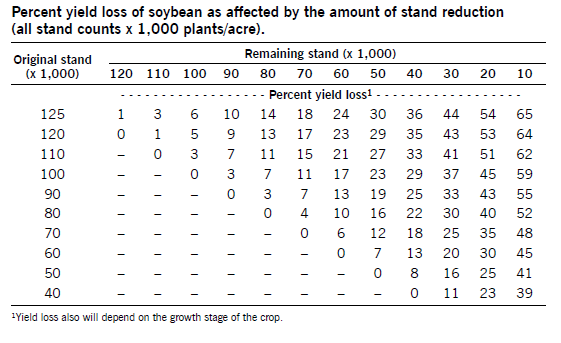
Even though early season soybean defoliation appears to be very devastating, research has shown that soybean plants can recover and yield fairly well under good growing conditions. The pod-setting and pod-fill periods are very susceptible to severe injury. Hail adjusters usually will defer final yield loss determinations until later in the season.
These tables and guides are being revised and updated continually as research becomes available. Specific loss predictions should be left to trained hail adjusters.
Source: National Crop Insurance Association - Soybean Loss Instruction - Pub. No. 6302.
Frost Damage
Soybean plants are damaged easily by frost in the 28 to 32 F range. Temperatures of 28 F for any extended period can completely kill soybean plants (stems and leaves).
During the early seedling stage (VE to VC), soybean has some tolerance to temperatures of 29 to 30 F for short periods. If the seedlings have been somewhat hardened off by cool temperatures for several days, then temperatures as cool as 28 F can be tolerated.
Once true leaves emerge, soybean plants become more susceptible to freezing temperatures below 32 F for any extended period. The unifoliolate leaf stage is slightly more frost-tolerant than the first or second trifoliolate leaf stages.
Late-season Frost Damage
Research information from Wisconsin has shown that all varieties tested had reduced yields when frost occurred at or before R6. Earlier-maturing varieties sustained economic yield losses from frost at more advanced growth stages than later-maturing varieties.
The greatest yield losses occurred when frost occurred at the R5 stage. The number of beans per plant and reduced bean size all contributed to overall yield loss. Maturity was hastened by some frost treatments and was not delayed in any of the trials studied.
Soybean seed on frost-damaged plants many mature and change color as early as or even earlier than nonfrosted soybean plants. The leaves tend to remain on the frost-damaged soybean plants. Seed moisture may be slightly higher and seed size usually is reduced as the soybean seed dries and shrinks.
A frost will not hurt soybean yields if the soybean growth stage is beyond R7. A frost between R6 and R7 may or may not affect yield, depending on the temperature and duration of the freeze. Beans that are still green and soft will shrivel.
Stalks rapidly turn dark green to brown and will not recover. Beans in pods that have turned yellow will mature normally. Some green beans will turn yellow after 30 to 40 days of storage.
Growers and researchers through the years have tried to use color keys such as yellow soybean leaves, yellow pods and brown pods to estimate soybean maturity and safety from frost. Generally, these methods did not work because of differences in varieties regarding symptoms of maturity. However, studies do show that “yellow” pods sprinkled with brown are the best clue of physiological maturity.
Open pods and check the shrinking of beans and look for separation of beans from the white membrane inside the pod. This indicates the soybean plants are physiological mature and fairly safe from frost injury. Pods do not all mature evenly.
Researchers have noted that if one or two pods on any of the upper four nodes have turned brown and other pods are light yellow to tan, the soybean plants are fairly tolerant to a killing frost. In the event of a leaf-killing frost when pods are still light green or yellow, wait until the pods are mature in color before combining. The most significant effect of an early frost on soybean may be in the reduction in their value as a future source of seed.
Soybean fields planted to narrow row spacings (6 to 12 inches) may have slightly more tolerance to light frosts than soybean planted in wider rows (30 to 36 inches). The heavy plant canopy of the solid-seeded, closely drilled beans tends to hold the soil heat better and, therefore, protects the plants to some degree.
Estimating Soybean Yields
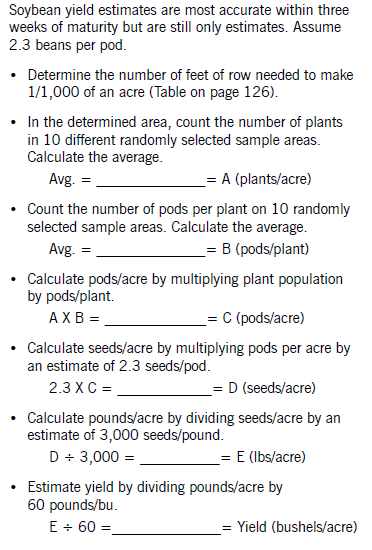
John Nowatzki, Extension Agricultural Machine Systems Specialist
Field studies in soybean harvesting have shown that a 10 percent or higher harvest loss is not uncommon, but studies also have shown that harvest loss can be reduced to 3 percent or less. To keep losses low, you need to know where harvest losses occur, how to measure loss, what is a reasonable level of loss, and the equipment adjustments and operating practices that will help reduce losses.
Soybean Loss
Soybean should be harvested when the bean moisture content reaches 13 percent on the first dry down (Figure 8). However, if beans are ready for harvest and then are subjected to alternating periods of wet and dry weather, preharvest or shatter loss can be high.
Figure 8. Shatter and machine loss related to moisture content at harvest.
Preharvest losses are influenced by the time of harvest and can be reduced by harvesting early. Preharvest losses are beans that have dropped on the ground prior to harvest.
Gathering or header losses can account for more than 80 percent of the total loss in soybean harvesting. These include all losses occurring at the header caused by actions of the cutter bar, reel and feeder auger, and losses from soybean left on uncut stubble.
Gathering losses are divided as follows:
• Shatter loss – shelled beans and detached bean pods that are shattered from stalks by the header and fall to the ground
• Stubble loss – beans remaining on stubble
• Stalk loss – beans in pods attached to stalks that were cut, fell on the ground and did not run through the combine
• Lodged stalks – beans remaining in pods attached to stalks that are lying on the soil or if cut, were cut at lengths larger than stubble height.
Soybean is an easy crop to thresh, separate and clean. The beans are easy to remove from the pod, and their size and shape make them easy to clean. But small errors in adjustment can cause serious bean loss.
Follow the settings recommended in the operators manual for cylinder speed and concave spacing, along with air flow and shoe settings. Then operate the combine in the field and check for loss. Usually, only small adjustments need to be made in the field.
Remember to run the cylinder at the slowest speed possible, but it must be fast enough to thresh the beans from the pods. Excessive cylinder or rotor speed is usually the main reason for cracked bean seeds.
Gathering Equipment
Several machinery developments have occurred to improve the soybean-gathering efficiency from using a conventional rigid cutter bar platform. These include the integral flexible floating cutter bar, the row-crop head, pickup finger reels, pickup guards, narrow-pitch knives (1½ vs. 3 inches) and combination pickup finger/air reels. These attachments provide significant reductions in soybean loss.
The flexible-floating cutter bar is able to follow soil slopes better and cut shorter stubble, reducing the number of pods left on the stalks. Pickup reels help lift lodged stalks so the cutter bar can slide under and retrieve them. Narrow-pitch knives help reduce side movement of plants and the resulting shatter loss.
Pickup guards ride on the soil surface and slide under lodged bean stalks so they are cut and directed into the combine header. A combination finger/air reel helps push cut stalks and pods back into the feeder housing to reduce bean pods and seed from building up on the cutter bar with the resulting shelling and bean loss.
Combine Adjustments
Reel speed and position are extremely important to reduce bean loss. Reels with pickup fingers will cause the least disturbance to the standing plants and, when positioned correctly, will place cut plants to convey smoothly along the header auger and into the feeder.
Proper reel speed is 25 to 50 percent faster than forward travel speed. Faster reel speeds will rip bean pods off stalks and result in increased bean loss. The axis of the reel should be positioned 8 to 12 inches ahead of the cutter bar.
Keep your ground speed to 3 miles per hour or less. Long stubble, uneven cutting height and shatter losses due to knife stripping are indications that the ground speed is too fast.
Operate the cutter bar as close to the ground as possible at all times. A floating flexible cutter bar with automatic header height control is essential for low loss levels.
Harvesting Quality Seed
Cylinder or rotor speed has more effect on seed damage than cylinder-concave clearance (Figure 9). Operate the cylinder only fast enough to remove the beans from the pods and no faster. Slowing the cylinder speed during the day as beans dry is important.
Figure 9. Relationship of cylinder speed to cylinder loss, germination and soybean crackage.
This is more important for conventional combines than rotary because Illinois tests found that rotary combines produced significantly fewer splits than conventional cylinder concave-type machines. But both types of machines easily could produce high-quality soybean. However, if cylinder or rotor speeds are too fast, damaged soybean will result.
Measuring Harvest Loss
Identifying where harvest losses are occurring is important so you can take measures to eliminate or minimize the loss. Soybean seed loss from the various areas are determined by making several seed counts inside a measured area.
The best way to measure the area is to form a 1-foot by 1-foot square. To do that, form a heavy piece of wire (No. 9 is good) into a square. You will use this square in the field to make seed counts.
Refer to Figure 10 and record your seed counts in the “Loss” table .
The procedure to use in the field is:
1. Operate the combine in the field and stop. Back up the combine about 20 feet.
2. Using the 1-foot-square frame, count all beans in the frame as you move the frame is across the width of the cutter bar. Refer to Figure 10.
a. Count beans in the uncut area to determine the “preharvest shatter loss.”
This is location 1 in Figure 10.
b. Count beans behind the combine to find the “total crop loss.”
This is location 2 in Figure 10.
3. Enter the bean counts for “preharvest losses” and “total crop loss” into the “Loss” table.
4. Divide that number by the number of frame counts that were completed across the cutting width of the combine. This number is the average number of beans per frame.
5. Divide No. 4 by 4 because approximately four beans per square foot equals 1 bushel per acre. If the beans you are raising are large, then three beans per square foot would equal 1 bushel per acre.
6. Subtract the preharvest loss from the total harvest loss. This is the machine loss due to combine operation. Then estimate your crop yield and divide the bu/acre loss by the yield. For example:
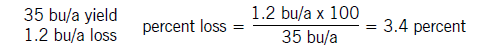
If the machine loss is more than 3 percent of crop yield, you may need to do further investigation into the source of loss.
7, Gathering loss is determined and measured between the combine header and the unharvested crop (location 3). Again, measurements should be made across the entire width of the header.
a. Determine shatter loss by counting all loose beans and beans in loose pods on the ground. Enter this number under shatter losses in the “Loss” table.
b. Determine loose stalk loss by counting all beans on loose stalks that were cut and are lying on the ground. Add this to the loss table.
c. Determine lodged stalk loss by counting all beans in pods on stalks still attached to the ground and lying flat. Add to the loss table. Pickup guards on the cutter bar may reduce this loss considerably.
d. Determine stubble loss by counting all beans in pods still attached to the stubble. Add this to the loss table.
Add the four gathering unit losses and subtract the preharvest loss from the gathering unit loss. This number will give you the gathering unit loss. Subtract the gathering unit loss from the machine loss to give you the cylinder and separation loss. Follow the step-by-step procedure in the “Loss” table.
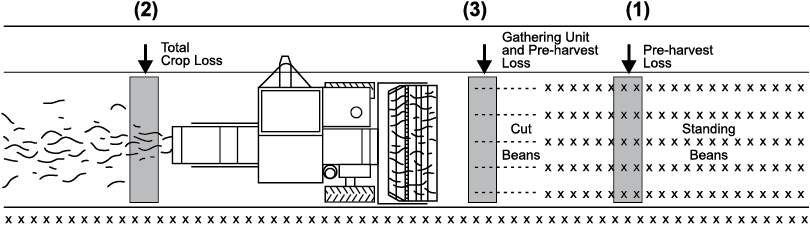
Figure 10. Location of areas to make seed loss counts.
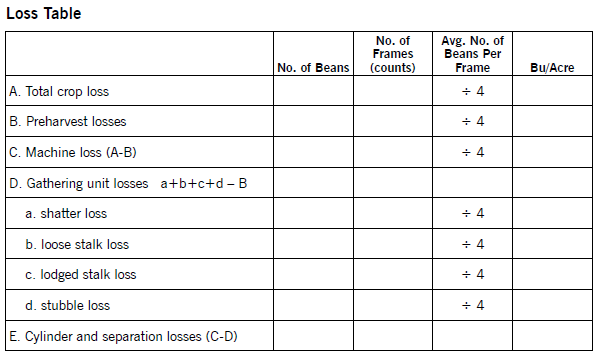
Soybean Drying, Handling and Storage
Kenneth Hellevang, Extension Engineer
Soybean usually is traded on a 13 percent moisture basis, so harvesting, storing and selling soybean as close to 13 percent moisture (wet basis) as possible is to the farmer’s advantage. Soybeans that is wetter than 13 percent moisture likely will mold under warm conditions, and buyers usually apply shrink factors and drying charges when wet beans are delivered.
On the other hand, soybeans that are drier than 13 percent moisture are more likely to split during handling. Because they weigh less, fewer pounds (bushels) are available for sale.
If the temperature of the stored beans is kept below about 60 F, soybeans usually can be held for at least six months at 13 percent moisture without mold problems. For storage under warmer temperatures or for storage times longer than six months, however, the recommended moisture content is 11 percent.
Oil quality also may be affected during storage at summer temperatures. One study showed that 12 percent moisture soybeans stored at 70 F had excessive oil quality loss in less than four months. During summer storage, soybeans need to be kept as cool as possible and at about 11 percent moisture to maintain storage quality.
Storage Management for 11 to 13 Percent Moisture Soybean
Soybeans that are harvested at 11 to 13 percent moisture can be placed directly into ordinary storage bins equipped with simple aeration systems (perforated ducts or pads and relatively small fans). The suggested winter storage temperature for grains and oilseeds in the upper Midwest is 20 to 30 F.
Because soybean usually is harvested at temperatures well above 30 F, cooling the beans by operating aeration fans during cool weather is necessary. Rather than waiting until outdoor temperatures drop to 20 to 30 F before cooling stored beans, you should cool them in 10- to 20-degree stages as average temperatures drop in the fall.
For example, if beans are harvested at 55 F, you could wait a few weeks until average outdoor temperatures drop to 40 F and run the fans long enough to cool all the beans in the bin to 40 F. Then shut the fan off for a few more weeks and repeat the cycle when the average outdoor temperatures fall to about 25 F.
The airflow provided by aeration fans usually is expressed as cubic feet of air per minute per bushel of beans, or cfm/bu. You can estimate the amount of fan operation time required to cool an entire bin of beans by dividing 15 by the airflow in cfm/bu.
For example, many on-farm storage bins have an airflow of about 0.2 cfm/bu, so the cooling time would be 15 divided by 0.2, or 75 hours, which is about three days. You can use this formula to estimate cooling time, but you should measure bean temperature at several different points in the bin to make sure that cooling is complete.
When you are operating aeration fans to cool beans that are 11 to 13 percent moisture, you don’t need to worry too much about relative humidity. Beans near the point where air enters the bin will rewet some during very humid weather and some overdrying will occur during very dry weather. But if fans are operated no longer than necessary to cool the bin, the overall moisture change will be quite small.
Changing the moisture of a crop takes about 50 times as long as changing its temperature, which means you can move a temperature front through 30 feet of beans by the time you’ve changed the moisture of a 7-inch layer.
If you are concerned about operating the fan during weather that is too humid or too dry, however, you can install controls that will operate the fan only during weather conditions that do not cause drying or rewetting. Keep in mind that these types of controls will limit the time that the fan operates, and cooling the entire bin will take more days than cooling it would without the controls.
Once soybeans have been cooled to 20 to 30 F, check the beans every two to three weeks during winter months to make sure that the temperature is stable and that no mold, insect and crusting problems are developing. If you find problems, or if the bean temperature has moved above or below the desired range, operate the aeration fan during 20 to 30 F weather to run a temperature front through the bin.
If you need to hold the beans into spring and summer, increase your frequency of checking the bins to every two weeks. Cover aeration fans to limit air blowing into the bin and warming the beans. Unless a problem develops, operating the aeration fans is not necessary. If you aerate during spring or summer to remove solar heat gain on the south wall or top of the bin, do so during the coolest weather available and make sure you keep the bean temperature at less than 60 F.
When spoilage problems develop in stored beans, they often start in pockets of accumulated fines (small pieces of broken seeds, weed seeds and stem material) and foreign material. This material is difficult to aerate and often is wetter and more susceptible to mold growth than are whole seeds.
Try to keep fines and foreign material out of the bin by setting combines for maximum cleaning or by running beans through a grain cleaner on the way into the bin. Other options are coring bins (removing some beans through the center unloading sump) after the bin is full, using a distributor to attempt to distribute the fines and material, or frequently moving spouts during bin filling.
Soybean Handling
Bean are subject to splitting during handling, so handle the beans gently. Belt conveyors, bucket elevators and drag or mass conveyors provide the gentlest handling. But normal grain augers can be used if they are operated slowly and fully, and pneumatic or air-type conveyors can be used if the air-to-grain ratio is set properly and if lines are laid out with a minimum number of very gradual curves.
Avoid long drop heights in bean handling by frequently adjusting the position of conveyors or by using bean ladders or other devices that break long drops into a series of shorter drops. One handler of food-grade soybeans recommends 10 feet as the maximum height for any single drop.
Artificial Drying
In most years, fall weather conditions in the upper Midwest will dry soybean to 11 to 13 percent moisture in the field. But in some years, weather conditions prevent soybean from drying to 13 percent moisture, and sometimes growers harvest at moistures greater than 13 percent to avoid the harvest losses that can occur at lower moisture contents.
Soybean can be harvested without too much damage up to about 18 percent moisture. If soybean is harvested at a moisture content above 13 percent, artificial drying is necessary.
Not much published research is available on soybean drying. Most drying recommendations are based on limited experience or are extrapolated from corn drying recommendations. In most cases, dryers that were designed for corn can be adapted for use with soybean.
Natural-air Drying
Using unheated air to dry soybean usually works well, but it is a slow process (four to six weeks, depending on initial moisture, airflow and weather). Bins used for natural-air drying should have fully perforated floors and large drying fans.
Fan power requirements depend on desired airflow and depth of beans. For example, delivery of 1 cfm/bu (cubic feet of air per minute per bushel of beans in the bin) through a 20-foot depth of soybean would require about 1 hp (horsepower) per 1,000 bushels of beans in the bin, while delivery of 1.5 cfm/bu through 20 feet of beans would take about 1.8 hp per 1,000 bu. An airflow rate of 1 cfm/bu through a bean depth of 26 feet also would require about 1.8 hp per 1,000 bu.
Management of natural-air soybean dryers is similar to that for natural-air corn dryers, except that soybean moisture values need to be about 2 to 3 percentage points lower than those recommended for corn. In southern Minnesota, use an airflow rate of at least 1 cfm/bu to dry 17 to 18 percent moisture beans, and 0.75 cfm/bu for up to 16 percent moisture beans.
In North Dakota and northern Minnesota, higher airflow rates are needed because fewer days are available for drying in the fall and outdoor temperatures are cooler. In northern areas, use at least 1 to 1.25 cfm/bu to dry soybeans that are 16 percent moisture or less, 1.25 to 1.5 cfm/bu for 17 percent moisture beans and 1.5 cfm/bu for 18 percent moisture beans. Estimated drying times using average October temperatures are shown in the following table.
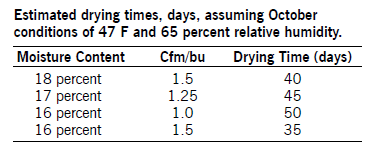
Low-temperature Drying
Early in the fall, especially in years with warm, dry weather, drying soybean to less than 13 percent moisture with no supplemental heat is possible. However, later in the fall, or in years with cool, damp weather, soybean might not dry to 13 percent, and adding a small amount of supplemental heat to the air in natural-air dryers might be helpful. Do not heat the air more than 3 to 5 degrees, though, or you will overdry the beans.
Some alternatives to adding supplemental heat to natural-air drying bins include:
• Turn off the fan when outdoor temperatures average below about 40 F in the fall, keep beans cold during winter and resume drying when average temperatures climb above about 40 F in the spring. Drying times and required airflow rates in the spring are similar to drying beans during October. Drying moisture contents greater than 15 percent is discouraged unless the airflow rate exceeds 1 cfm/bu.
• Install bigger fans so you can finish drying earlier in the fall when the weather is better.
• Use manual or automatic controls to turn off the fan during periods of high humidity. Fan control will increase the number of days required for drying, but it will result in drier beans.
High-temperature Drying
Many kinds of gas-fired corn dryers can be used to dry soybean, but beans can be damaged and you run the risk of dryer fires. Soybean splits easily if the beans are dried too fast or are handled roughly. Set the drying air temperature according to the manufacturer’s recommendation and monitor bean quality.
Column-type dryers often can be operated at about 120 F without causing too much soybean damage, although some trial and error might be required to set dryers properly. Examine beans leaving the dryer carefully and reduce the temperature if you’re getting too many splits.
Research has shown that exposing beans to relative humidities of less than 40 percent can cause excessive splitting. For every 20 degrees that you heat air, you cut its relative humidity approximately in half, so you don’t need very much heat to produce relative humidities less than 40 percent.
If the soybeans will be saved for seed, keep drying temperatures under 110 F to avoid killing the embryo. Food-grade soybeans normally cannot be dried in a high-temperature dryer.
Don’t forget that crops dried in gas-fired dryers must be cooled within a day or so to remove dryer heat. This can be done in the dryer or in aerated storage bins. Stored beans should be aerated again later in the fall to cool them to 20 to 30 F for winter storage.
Immature, Frosted or Green Beans
In years when frost kills soybean plants before the seeds are fully mature, make sure you remove as much chaff and green plant material as possible before binning the beans. Small percentages of immature beans can be stored without significant molding if blended with dry beans, but concentrations of green beans and chaff can lead to heating and spoilage in storage.
Reconditioning Overly Dry Soybean
In years with exceptionally warm, dry falls, soybean sometimes is harvested at moisture contents well under 13 percent moisture. Although adding water to increase soybean moisture is illegal, given enough time and a high enough airflow per bushel, increasing the moisture content of soybean is possible by aerating the beans with humid air. But here are some practical concerns and limitations:
• The process is quite slow, even with the high airflow per bushel (0.75 to 1 cfm/bu) available on bins equipped for drying. It will be similar to the speed of drying.
• Fan control is critical, and some beans could end up too wet for safe storage.
• You may end up with layers of wet beans and dry beans unless you can find some way to mix them in the bin or while unloading the bin.
• Swelling that accompanies rewetting will increase stress on bin walls and can damage the bin wall. Periodically unloading some beans from the center of the bin has been reported to reduce the bin wall pressure.
The table of equilibrium moisture values shows the moisture content that soybeans would reach if exposed to different combinations of temperature and relative humidity for long periods of time. If you continuously aerated a bin of beans, they would tend to lose moisture during periods of low humidity and gain moisture during periods of high humidity.
To recondition beans to 13 percent moisture during normal fall temperatures of 30 to 60 F, you would need to control the fan so that it operates during weather that has an average relative humidity of 65 to 70 percent. The table indicates that bean moisture increases sharply as relative humidity increases, which means that rewetting a layer of beans to a moisture content that is too high for safe storage is quite easy.
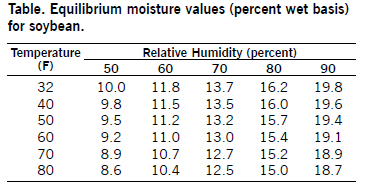
During reconditioning, the moisture of the whole bin doesn’t change at once. A rewetting zone develops and moves slowly through the bin in the direction the airflow is moving. This is similar to the way a drying zone moves through a drying bin.
In most cases, not enough high-humidity hours are available in the fall to move a rewetting zone all the way through the bin. And in many cases, depending on how the fan is controlled, the parts of the bin that have been rewetted will be too wet for safe storage. Your best option is to mix the wet layers with the dry layers to reduce the spoilage risk and avoid drying charges for the wet layers when the beans are sold.
Mixing can be accomplished to a limited extent by emptying the bin and moving the beans through a grain-handling system. The most effective way to mix the beans, though, would be to use an in-bin stirring system.
If the initial moisture content of the beans is 10 percent or less, controlling the fan so that it only runs when relative humidity of the air reaching the beans is greater than about 55 percent should result in rewetting. If you use a single humidistat to turn the fan on anytime humidity is greater than 55 percent, average humidity during the hours the fan operates should be well above 55 percent, and the beans are likely to rewet to at least 13 percent. Because humidity is almost always higher at night than it is during the day, an alternative to a humidistat would be a timer set to run the fan only during nighttime hours.
If you aren’t equipped to mix beans after reconditioning, you need to avoid rewetting them to moisture levels that are too high for safe storage. Approaches to prevent excessive rewetting include:
• Reducing the humidity setting on the humidistat that controls the fan so the fan runs during drier conditions
• Adding a second humidistat that stops the fan when relative humidity reaches very high levels
• Installing a microprocessor-based controller that monitors temperature and humidity and only runs the fan when air conditions will bring the crop to the desired moisture content (for either drying or rewetting)
The disadvantage of the last two approaches is that the fan doesn’t run as many hours as it would with a single humidistat control and less total moisture would be added. Reconditioning time depends primarily on airflow per bushel and weather conditions. It is fastest when airflow per bushel is high and the air is warm and humid.
Reconditioning will be the most successful in a bin equipped as a drying bin - one that has a fully perforated floor and a fan that can deliver at least 0.75 cfm/bu. Even with this airflow, you probably would need at least a month of fan operation to move a rewetting front all the way through the bin. And keep in mind that you can’t run the fan continuously because in a typical fall, continuous fan operation would result in drying rather than rewetting.
Beans swell when they absorb moisture, and this may result in enough pressure to rupture bin walls. Periodically unloading some beans from the center of the bin has been reported to reduce the bin wall pressure. Also, using a vertical-stirring auger to mix layers of dry and wet beans might reduce outward pressure generated during rewetting.
To increase chances of success in using airflow to recondition soybean:
• Use a bin equipped with a fully perforated floor and a fan that can deliver at least 0.75 cfm/bu.
• If it is available, use a bin equipped with stirring equipment. If stirring equipment is not available, consider transferring the beans to another bin to mix the wet and dry layers.
• Use timers, humidistats, programmable controllers or some other type of automatic control to limit fan operation to weather conditions that will cause rewetting.
• Keep reconditioned beans cool (20 to 30 F is the suggested winter-storage temperature in the upper Midwest) to reduce chances of spoilage.
• Watch carefully for signs of moldy beans and excessive stress on the bin.
Food or High-value Soybean
More care is required with high-value soybean primarily to minimize the amount of cracked skins and beans. The optimum moisture content for harvest is about 13 to 15 percent. At moisture contents below about 13 percent, you have more split beans, germination is reduced due to more damage during harvest and handling, field loss will be greater and less weight is available to market below 13 percent moisture.
To minimize damage during handling, belt conveyors are preferred to augers. If augers are used, they should be operated at a slow speed and be kept full. Pneumatic conveyors can be used if the proper air-to-grain ratio is maintained, gentle curves are used in the conveying tubes and a low conveying velocity is used. Drop heights should be minimized, so bean ladders are recommended in bins or other locations where the beans may fall during conveying.
Cold beans are more susceptible to damage during handling. Use care in drying beans because much of the damage that occurs during handling is due to stresses created during drying.
Damage during drying occurs when the relative humidity of the drying air is below about 40 percent. Generally, the drying air in a high-temperature dryer should not be heated more than 20 degrees to minimize the potential for damage to the seed coat.
One study showed that with a 100 F drying temperature, 10 to 60 percent of the skins were cracked and 5 to 20 percent of the beans were cracked. Another study showed about 15 percent of the seed coats cracked with the drying air at 40 percent relative humidity, and 30 percent of the seed coats cracked with a 30 percent relative humidity.
U.S. Standards for Soybean Grades and Grade Requirements
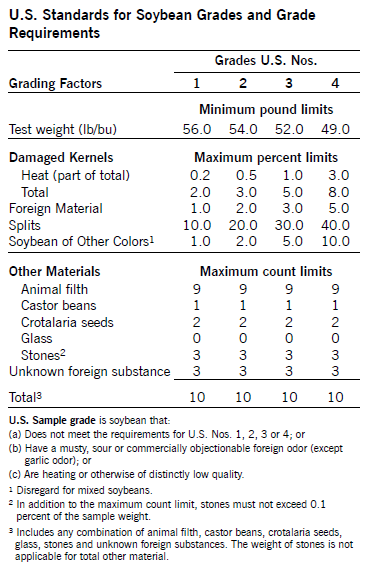
Contributors to Soybean Production Field Guide
• Greg Endres, agronomist, NDSU Extension
• David Franzen, soil science specialist, NDSU Extension
• Kenneth Hellevang, agricultural engineer, NDSU Extension
• Joseph Ikley, weed specialist, NDSU Extension
• Hans Kandel, agronomist, NDSU Extension
• Janet Knodel, entomologist, NDSU Extension
• Dean Malvick, plant pathologist, UMN Extension
• Sam Markell, plant pathologist, NDSU Extension
• Berlin Nelson, plant pathologist, NDSU
• John Nowatzki, ag machine systems specialist, NDSU Extension
Other contributors
• Pat Beauzay, NDSU Entomology
• Duane Berglund, agronomist emeritus, NDSU Extension
• Ted Helms, soybean breeder, NDSU
• Rich Zollinger, weed specialist emeritus, NDSU Extension
Resource Publications
North Dakota State University
• “North Dakota Soybean Variety Trial Results and Selection Guide” (current year) (A843)
• “North Dakota Weed Control Guide” (current year) (W253)
• “North Dakota Field Crop Plant Disease Management Guide” (current year) (PP622)
• “Crop Rotations for Increased Productivity” (EB48)
• “Soybean Soil Fertility” (SF1164)
• “Soybean Aphid, Aphis glycines, Management in North Dakota” (E1232)
Useful Soybean Websites
Soybean Production Management
NDSU Research Extension Centers
NDSU Insect Updates for North Dakota
North Dakota Crop Improvement and Seed Association
Commodity Groups and Organizations
Soybean – General

Nodules on soybean roots
(Courtesy D. Berglund)

Soybean flowers
(Courtesy D. Berglund)
Bean development stages (Courtesy D. Berglund)

Iron chlorosis deficiency (Courtesy D. Berglund)

Soybean pod 10 mm long
(Courtesy D. Berglund)

Harvest loss: four beans per square foot is 1 bu/a
(Courtesy D. Berglund)
Soybean – Insects

Armyworm adult
(Courtesy G. Fauske, NDSU)

Bean leaf beetle adult
(N. Wright, Florida Department of Agriculture & Consumer Services, Bugwood.org)

Dingy cutworm adult
(Courtesy G. Fauske, NDSU)

Armyworm larva
(Courtesy R. Smith, Auburn University, Bugwood.org)

Bean leaf beetle defoliation
(Courtesy C. Strunk, South Dakota State University, Bugwood.org)

Dingy cutworm larva
(Courtesy J. Gavloski, Manitoba Agriculture, Food and Rural Initiatives)

Redbacked cutworm adult
(Courtesy G. Fauske, NDSU)

Darksided cutworm adult
(Courtesy G. Fauske, NDSU)

Green cloverworm larvae
(Courtesy J. Gavloski, Manitoba Agriculture, Food and Rural Initiatives)

Redbacked cutworm larvae
(Courtesy J.Gavloski Manitoba Agriculture, Food and Rural Initiatives)

Green cloverworm adult
(Courtesy J. Gavloski, Manitoba Agriculture, Food and Rural Initiatives)

(Courtesy J. Gavloski, Manitoba Agriculture, Food and Rural Initiatives)

Painted lady butterfly
(Courtesy W. Ciesla, Forest Health Management International, Bugwood.org)

Alfalfa webworm adult
(Courtesy W. Cranshaw, Colorado
State University, Bugwood.org)

Grasshopper adult
(Courtesy P. Beauzay, NDSU)

Thistle caterpillar
(Courtesy J. Knodel, NDSU)

Alfalfa webworm larva
(Courtesy J. Knodel, NDSU)

Grasshopper nymph
(Courtesy G. Fauske, NDSU).

Potato leafhopper adult
(Courtesy S. Brown, University of Georgia, Bugwood.org)

Soybean aphids (adults, nymphs, and white casted skins) on underside of leaf
(Courtesy P. Beauzay, NDSU)

Seedcorn maggot adult
(Courtesy Pest and Diseases Image Library, Bugwood.org)

Potato leafhopper nymph
(Courtesy S. Brown, University of Georgia, Bugwood.org)

Close-up of soybean aphid alate
(Courtesy P. Beauzay, NDSU)
Seedcorn maggot larva
(Courtesy W. Cranshaw, Colorado State University, Bugwood.org)

Close-up of two-spotted spider mite
(Courtesy D. Cappaert, Michigan State University, Bugwood.org)

Webbing from two-spotted spider mites
(Courtesy D. Cappaert, Michigan State University, Bugwood.org)

Click beetles (adult wireworm)
(Courtesy S. Brown, University of Georgia, Bugwood.org)

Stippling injury from two-spotted spider mites
(Courtesy W. Cranshaw, Colorado State University, Bugwood.org)

Wireworm
(Courtesy M. Boetel, NDSU)
Soybean – Diseases

Phytophthora root rot
(Courtesy H.A. Lamey)
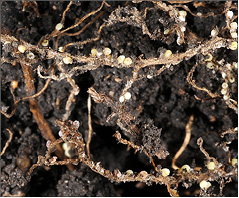
Soybean cyst nematode
(Courtesy S. Markell)

Fusarium root rot.
(Courtesy B.D. Nelson)

(Courtesy B.D. Nelson)

Rhizoctonia root rot
(Courtesy B.D. Nelson)

Sudden death syndrome
(Courtesy B.D. Nelson)

Bacterial blight
(Courtesy L. Prom)

Downy mildew on upper leaf
(Courtesy S. Markell)

Brown stem rot
(Courtesy C. Grau)

Septoria brown spot
(Courtesy D. Malvick)
Pod and stem blight
(Courtesy USDA)

Leaf puckering from virus
(Courtesy Illinois Ag. Exp. St.)
Seed mottling from virus
(Courtesy H. J. Johnson)

Bacterial pustule (soybean rust look-alike)
(Courtesy S. Markell)

Charcoal rot
(Courtesy D. Jardine, Kansas State University)

Soybean rust through hand lens
(Courtesy S. Markell)

Leaf distortion from virus (Courtesy M.C. Shurtleff)

Stem canker
(Courtesy D. Malvick)


