Water Quality and Nitrogen (AE1216, Reviewed June 2017)
Availability: Web only
Condition of our Water Resources
Groundwater
A nationwide survey conducted by EPA showed that 1.2 percent of community and 2.4 percent of private drinking water wells exceeded the 10 parts per million (ppm) nitrate-nitrogen (NO3-N) standard. About 20 percent of the drinking water wells in the Canadian prairie provinces exceeded 10 ppm NO3-N. North Dakota studies indicate that the percentage of water wells that exceed 10 ppm NO3-N ranges from 2 to 14 percent. Review of North Dakota Health Department data and information from Manitoba indicate that the frequency of occurrence of high concentrations of NO3 in water wells has not changed substantially in much of the northern prairie region since well monitoring data collection began in the 1940s.

Surface water
Monitoring during the 1990s by the USGS of selected U.S. watersheds (NAWQA study) has revealed relatively low concentrations of N in surface water. In the Red River basin NO3-N was less than 10 ppm but was high enough in some cases to contribute to eutrophic conditions. Monitoring along eight major rivers in North Dakota (Cannnonball, Heart, James, Knife, Missouri, Red, Sheyenne, and Souris) revealed concentrations less than 1 ppm NO3-N. The interim standard for Class I streams in North Dakota is 1 ppm for NO3-N. Since the beginning of surface water monitoring in North Dakota, no substantial changes in NO3-N concentrations have been detected.
Although long-term trends in N concentrations in northern prairie wetlands, lakes, and tributaries have not been detected, seasonal changes are regularly observed. The highest concentrations of NO3-N are found during spring runoff. When temperatures increase during late spring and summer, NO3-N lowers as biological activity increases. However, NO3-N by itself tells only part of the story, since the major portion of N in surface water is present in organic compounds. Total-N (NO3 + NH3 + organic N) is highest in the summer during the height of biological activity but decreases substantially in the fall when temperatures decline and organisms die. In the winter, total-N generally increases, because NH3 is released from decaying biologic material on the bottom.
The effect of N on aquatic organism growth and eutrophication is intertwined with the nutrient phosphorus (P). Both N and P are nutrients required for biologic growth. It is generally accepted that in a lake environment a balanced system has an N to P ratio of 10:1. When the ratio is less than 10, N is the limiting nutrient with respect to biologic growth. In the northern prairies ratios less than 10 are often observed due to the high amount of P contained in the native soils. Just as N is subject to seasonal variations in water resources, so is the N:P ratio. Another complication to the relationship between biologic growth and the N:P ratio is organisms, such as blue-green algae, that extract N from the atmosphere. A low N:P ratio won’t impede the growth of these organisms.
Where does the nitrogen in our water come from?
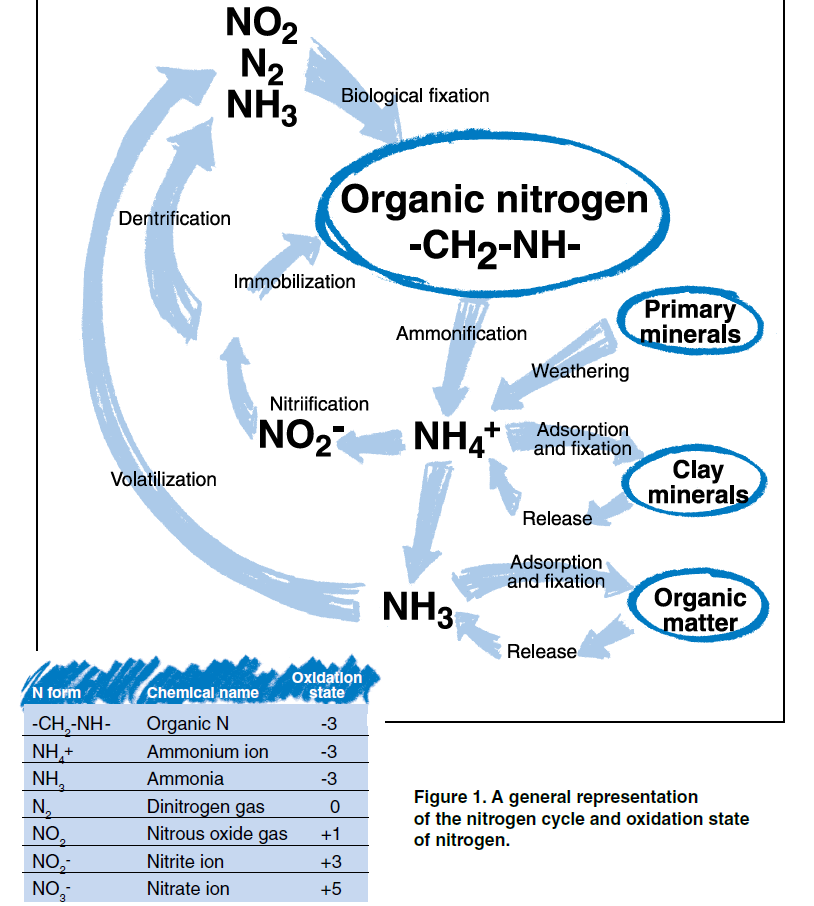
N is present in the air, water, soil, rocks, plants and animals. N is present in many different forms and is continually changing from one molecule to another and moving from one place to another. This process of change and movement is commonly referred to as the N cycle. The most active zone of N exchange is within and among living organisms on the earth’s surface. The earth’s geologic and soil materials compose the least active zone of N exchange but account for 98 percent of the total amount of N on the planet. Although it is a relatively small portion of total N, the atmosphere is composed of 78 percent gaseous N. This is equivalent to 35,000 tons of N above every acre of land.
Mineralization and Immobilization
N is an essential component of many carbon-based or organic molecules, such as proteins in biologic tissues. When the carbon (C) in organic materials is used for energy by microorganisms, N is released in a molecular form that is no longer associated with C. This process of transformation from organic to inorganic N is called mineralization. Ammonification is the first step in mineralization that produces ammonium (NH4). Nitrification is the second step that converts NH4 to nitrate (NO3). The opposite of mineralization is immobilization, the process of inorganic N uptake by microorganisms. Both of these processes occur simultaneously and provide continuous movement between the organic and inorganic pools of N in the environment.
Ammonification occurs when microorganisms extract energy from organic materials and release the byproducts carbon dioxide (CO2) and NH4 ions. Nitrification occurs when other microorganisms in the soil extract energy from NH4 and release the byproduct nitrite (NO2). The final step of nitrification occurs when another group of microorganisms uses NO2 for energy and the byproduct is NO3. NO3 is the form of N that is most easily taken up by plants and is commonly applied to fields as crop fertilizer.
The balance between mineralization and immobilization determines whether NH4, NO2, and NO3 are present in the soil. Agricultural practices that favor mineralization will enhance plant performance by increasing the amount of NO3 in the soil; however, if the NO3 is not used efficiently the potential for N loss to water resources increase. Practices that favor immobilization will provide greater environmental protection but do not provide the benefit of available N for plant growth.
Management rules of thumb
■ Low temperatures and dry soil conditions slow the rate of mineralization and immobilization.
■ Incorporation of plant residue into moist soil enhances the rate of microbial decomposition.
■ High carbon plant residues (greater than 20:1 C/N) favor immobilization and release available N much slower compared to residues with less carbon.
■ High soil pH and low cation exchange capacity inhibits nitrification, which may lead to high levels of NH4 and nitrites.
■ Mineralization generally does not provide enough N for modern crop varieties to meet their genetic yield potential.
Fixation and Adsorption
Biological fixation of N is the process used by some microorganisms to extract elemental N gas (N2) from the atmosphere. Bacteria such as azotobacter and clostridia fix N in the soil environment. Blue-green algae fix N in aquatic environments. Much of the stored N in soils has been fixed from the atmosphere through the symbiotic relationship between certain N fixing bacteria and specific plants. Most notable is the relationship between Rhizobia bacteria and legume plants. The bacteria invade root hairs of the host plants where plant nutrients are exchanged for NH4 produced by N fixation.
The NH4 ion, with its positive charge, is adsorbed to negatively charged surfaces of particles of clay and organic matter in the soil. Adsorbed NH4 is available to microbes and plants but is essentially immobile with respect to water movement. NH4 is defined as “fixed” when adsorption occurs in the interlayer space of clay minerals and held so tightly that it is unavailable for plant use or exchange with soil water. Fixed NH4 may be released at a very slow rate. The potassium ion (K) is fixed similarly to NH4 and affects the capacity for NH4 fixation. If K has been previously introduced and fixed, its presence in the interlayer space of clay minerals will not allow subsequent fixation of NH4 to occur.
With respect to water resources, N fixation and adsorption have both positive and negative consequences. Both fixation and adsorption store N in the soil environment in a form that is relatively immobile. Potential for fixed or adsorbed N to move to water resources is quite low. However, fixed nitrogen may be mobilized through mineralization and weathering. It is particularly important to account for nitrogen inputs from legume crops, where under some extreme circumstances as much as 500 lbs/acre/year of N may be fixed. If the release of fixed N is not considered when managing nutrient inputs for plant growth, N fertilizer will be applied in excess of plant needs. Excess application of N fertilizer increases contamination potential of both groundwater and surface water. Weathering of geologic materials with fixed NH4 will release nitrogen to the surrounding environment. Elevated NO3 in groundwater due to oxidation of fixed NH4 from shaley sediments can occur in the northern plains.
Management rules of thumb
■ Efficient use of applied N fertilizer must account for the amount of available N previously fixed by legume crops such as alfalfa, clover, and beans.
■ Soils with higher clay contents have greater capacity to adsorb and possibly fix NH4.
■ The process of NH4 fixation is related to the amount of fixed potassium. Soils high in potassium are less likely to fix NH4.
■ Shrink-swell clays such as montmorillonite do not fix NH4 if water is present within the interlayer space of the clay particles; however, water removal through drying or freezing will allow fixation to occur.
■ Fixed NH4 usually increases with depth in the soil profile and may be found in large quantities in geologic materials.
Gaseous Losses of Nitrogen
Some forms of N are removed from the soil by escape to the atmosphere, or volatilization. The process of denitrification converts NO3 to the gaseous forms of nitrous oxide (N2O) and elemental N (N2). This process removes N from wet or anaerobic soils as these gases volatilize.
NO3 in aquifers can also be converted to gaseous forms of N that can be a positive factor in removing NO3 from groundwater. Denitrification also depends on the presence of a source of energy for the denitrifying bacteria. Organic carbon usually serves as that source of energy, and absence of carbon is often the limiting factor slowing denitrification in soils or groundwater.
Another volatile form of N that may be lost from the soil is ammonia (NH3). During the ammonification step of mineralization, losses of NH3 may occur, particularly from organic materials with greater than 2 percent N. On a global scale, NH3 volatilization from domestic animal waste contributes 3 to 5 times the amount of N to the atmosphere compared to combustion of fossil fuels.
Conversion to gases that are lost to the atmosphere is a natural mechanism that helps protect water resources from excessive levels of NO3. This process may be particularly important in protecting aquifers in the northern prairies. It is also an active process in removing N from wetland and riparian systems. Maintenance of wet soils on northern prairie landscapes is required to retain this buffering mechanism.
Loss of NH3 to the atmosphere also functions to remove N from the system. These losses help reduce potential movement of N to surface water via runoff and erosion. However, upon contact with droplets of water some of the NH3 is returned to the earth in precipitation. Atmospheric deposition of NH3 is measurably greater in the vicinity of large animal feeding operations and may be an important source of N for surrounding surface water resources.
Management rules of thumb
■ Preserving the natural function of wet soils to denitrify N may be accomplished by maintaining wetland and riparian area integrity. Establishing vegetative buffers on the borders of these areas will help reduce encroachment of other activities that diminish their function as an environmental filter.
■ Because carbon is the most important energy source that drives the denitrification process, carbon preservation on the landscape through the application of soil conservation practices is critical.
■ NH3 losses from animal waste can be reduced by utilizing it for its nutrient value rather that storing it and allowing volatilization to occur. When applied as fertilizer, further loss of NH3 can be reduced substantially by incorporating the waste into the soil and avoiding application during hot days and on high pH soils and sandy soils with low cation exchange capacities. 
Water transport
The combination of the chemical processes described above with the physical processes of water movement determines the impact of N on water resources. Because N occurs in so many different chemical forms, the various processes of water transport provide a range of impacts with respect to water resources. N is transported in precipitation, groundwater flow, and surface water runoff.
Precipitation
N in precipitation occurs as NH3 and NO3. Precipitation adds more N to the Earth’s surface in the tropical regions than it does in more arid regions like the northern plains of the United States. The United States Geological Survey recently estimated that the amount of N added to the soil or water surface in the Red River watershed is approximately 1.3 pounds per acre per year.
Management rules of thumb
■ Under most agricultural settings atmospheric deposition of N is only a small component of the total input of N and is generally not an important factor with respect to crop yields.
■ If large animal feeding operations are within a few miles, atmospheric deposition of N may be high enough to be considered in the overall management of N inputs

Groundwater flow
The NO3 form of N is soluble in water and mobile in soil, while NH4 is adsorbed to soil particles and immobile. NO3 dissolved in water can readily leach through the soil profile into groundwater. Leaching is minimal in semi-arid areas, particularly in fine and medium textured soils. No leaching beyond the root zone is likely to occur during the active growing season unless there is excessive rain. The greatest potential for NO3 leaching to groundwater occurs in the spring on coarse textured soils with shallow water tables. Spring snow-melt and rains combined with minimal crop water use contribute to wetter soils and greater gravitational water flow through the soil profile.
Management rules of thumb
■ Although leaching is not as prevalent in the northern plains as compared to other agricultural regions, deliberately carrying excess NO3 in the soil from fall to spring is not a recommended practice.
■ Although N is generally applied as NH3, it is readily nitrified to NO3 at temperatures greater than 45o F and available for plant uptake and leaching. NH3 is only stored in the soil during the winter months, so it remains important to match NH3 input with crop requirements on an annual basis.
■ It is particularly important to avoid leaving excess NO3 in soils with sandy or gravelly textures because of the high potential for leaching.
Surface water runoff
Sediment in runoff water is the most common pollutant of surface water. Attached to the sediment is a certain amount of organic N and NH4. NO3 is moved with the runoff as a soluble form of N. Movement of sediment is influenced by many factors that were first accounted for in the Universal Soil Loss Equation (USLE) used by the Natural Resources Conservation Service (NRCS) to predict soil erosion by water. The USLE has been subsequently revised to improve its predictions, but in general the factors remain the same. Usually a greater percentage of eroded sediment is delivered to streams in smaller watersheds with steep slopes and fine textured soils. Many of the soil characteristics that contribute to lower productivity, such as steep slopes, high erodibility, and shallow restrictive layers, also contribute to greater impacts to water resources from erosion.
Estimating nutrient delivery to streams and lakes using USLE predictions of soil erosion cannot be done without other information, because only a portion of the eroded sediment actually reaches a water body. In addition water quality problems are often associated with critical areas, so erosion estimates for the whole watershed are poorly correlated with actual contaminants reaching the stream or lake. Also, water quality problems are related to the soluble forms of nutrients that the USLE was not designed to consider. When using erosion predictions from the USLE to determine water quality in streams and lakes, always keep in mind that the USLE was designed to determine how much material may be removed from fields, but not how much may actually be delivered to streams and lakes. Accurate estimates of N inputs to water resources are further complicated by the many biologic processes that consume or release N as it is being transported.
Management rules of thumb
■ Conservation practices that control erosion are effective in protecting streams and lakes from water quality problems related to sediment, but direct correlation between erosion losses and water quality parameters are difficult to demonstrate.
■ Effective surface water protection must include land management practices designed to also reduce the movement of soluble forms of nutrients. Much of the sediment load is deposited at the base of hills, but the soluble load moves with runoff to streams and lakes.
■ Identifying and managing critical areas within a watershed is particularly important to meet water quality goals. Although many soils within a watershed benefit from the erosion protection provided through the application of conservation practices, the benefit to water quality will depend on whether active contaminant sources are contained or limited.
■ Fertilizer that is incorporated into the soil is not available for movement with surface runoff. The benefits of disturbing the soil surface to incorporate fertilizer and reduce soluble nutrient losses need to be balanced with the benefits of reduced tillage in controlling losses of nutrients attached to sediment. Different methods of incorporation should be considered. Subsurface injection or banding of fertilizer provide 100 percent incorporation and will cause much less disturbance of the soil surface compared to using tillage to mix surface applied fertilizer.
Further Information and References
For information related to nitrogen and water quality refer to:
AE1218 “Working to avoid nitrogen contamination”
AE1217 “How to assess for nitrogen problems in water resources”
EB-64 “Managing nitrogen fertilizer to prevent groundwater contamination”
For an in-depth discussion of nitrogen and water quality refer to:
ER-62 “Diffuse sources of nitrogen related to water quality protection in the Northern Great Plains”
Reviewed June 2017
Illustrations by Ag Comm


