Sodicity and Remediation of Sodic Soils in North Dakota (SF1941, Nov. 2019)
Availability: Web only
Sodicity, according to Natural Resources Conservation Service (NRCS), is the degree to which a soil is affected by sodium (Na), expressed as the sodium adsorption ratio (SAR) of sodium ions (Na+) to the total of calcium ions (Ca+2) and magnesium ions (Mg+2) from a saturation extract. The formula is (Na+)/√((Ca+2 + Mg+2)/2), where units of each cation are millimoles per liter (mmol(c)/L). A value for SAR also can be closely estimated through using the percent of the Na value within the soil base exchange capacity.
The effect of sodicity on crop production and soil condition is very different than the effect of soluble salts. Although sodium enters the soil solution as a salt, sodium is attracted to negative charges of organic matter and particularly soil clays, and becomes a part of the cation exchange capacity.
In a calcium-dominated soil, clay particles group together in an orderly fashion, resulting in avenues between the particle groups (aggregates) through which water can flow and roots can grow (Figure 1).
In a sodium-dominated soil, the clay particles become randomly distributed or dispersed, leading to swelling when wet and sealing when dry, with few avenues for water to penetrate and roots to grow.
The application of soluble calcium amendments may be used to improve soil physical properties due to 1) an increase in electrolyte concentration (salinity) (Figure 2) and 2) displacement of that displacement Na+ by Ca+2 on the cation exchange sites. The displacement of Na+ by Ca+2 is important; however, the salt concentration (EC) of the soil water is the most crucial factor affecting the stability of soil structure.
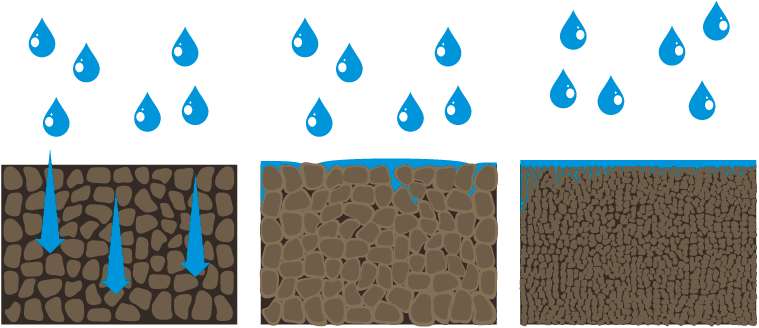
Figure 1. Illustration of the flocculation in a calcium-dominated soil, compared with swelling and dispersion in a sodium-dominated soil. (NDSU)
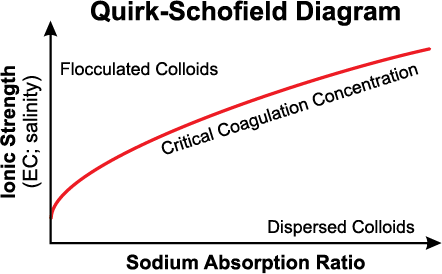
Figure 2. Relationship between sodium adsorption ratio (SAR) and soil water ionic strength (EC). The negative effects of sodium on soil properties (swelling and dispersion) may be reduced with greater EC.
Flocculation, swelling and dispersion happen due to the chemical properties of sodium and calcium. Sodium ions are satisfied when they have a full array of water surrounding each of them (Figure 3).
The charge configuration of calcium results in binding together clay particles (Figures 3 and 4), whereas the hydration property of sodium leads to clay dispersion when sodium reaches a critical concentration.
Figure 3. Illustration of fully hydrated sodium ion (left) and coordinated calcium ion (right).
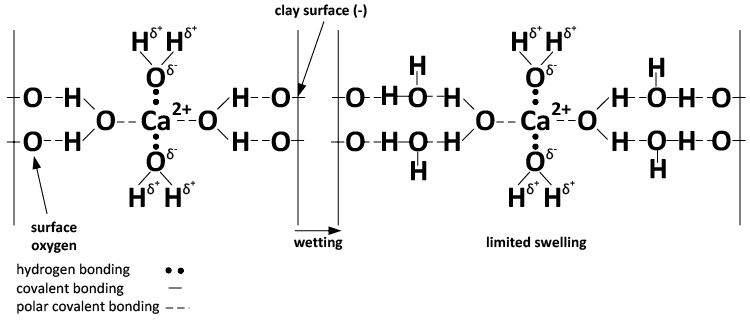
A) Ca-dominated system.
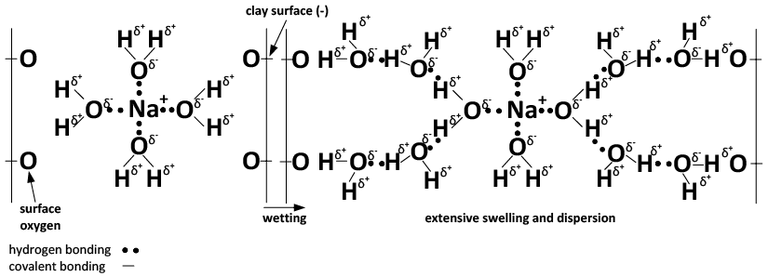
B) Na-dominated system.
Figure 4. Chemical depiction of the effect of Ca (A) and Na (B) and wetting on soil swelling and dispersion. (Adapted from Rengasamy and Sumner, 1998)
Dispersion
The video shows a cube of sodic soil in water (left) and a cube of calcium-dominated soil (right) and the dispersion that takes place through time.
Swelling
Although when dry, sodic soils become quite hard and difficult to seed into or till, they wet readily and the soil swells. When wet, the soil has no “bottom” and farm implements can become stuck easily when the nonsodic soils surrounding them are traveled (Figure 5).
A relationship exists between the swelling properties of sodic soils due to the SAR and soluble salt concentration (EC). In a study using a North Dakota sodic soil with SAR of 14, an EC value less than 1 resulted in a soil field capacity water content of about 32% (Figure 6). The field capacity water content at an EC of 4 was about 25%.
In general, dispersion and swelling are possible when the SAR is greater than 5 and EC is less than 1.5 millimhos per centimeter (mmhos/cm). Maintaining an EC that will prevent dispersion and swelling from sodium is, therefore, part of the management plan for managing crop productivity in sodic soils. As the SAR increases with a constant EC, the water content of the soil at field capacity increases (Figure 7).

Figure 5. Wetness of a sodic soil near Wyndmere, N.D. (NDSU photo)
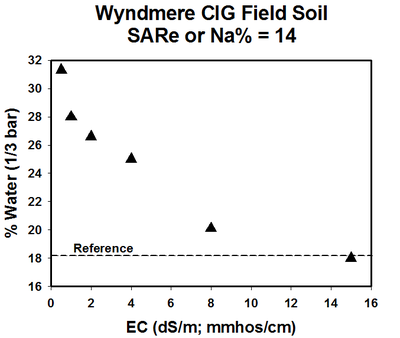
Figure 6. Water content at field capacity as affected by soil EC. (He and DeSutter, unpublished data, 2015)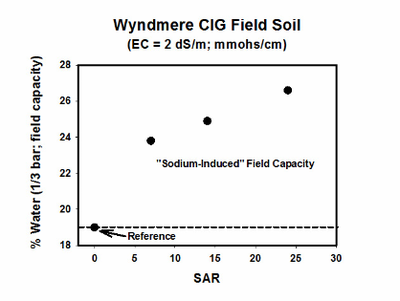
Figure 7. Water content at field capacity as affected by soil SAR. (He and DeSutter, unpublished data, 2015)
Restrictions to Crop Productivity
Soils with excess sodium can develop a natric horizon (Figure 8). The natric horizon is nearly always within 12 inches of the soil surface, which greatly restricts rooting depth. A few roots can follow the outside of each natric column, but sometimes the columns are wider than those in Figure 8, and little nutrition or water is taken up from depths under the natric horizon.
When wet, the soil swells and seals the soil from water movement through the columns, and when dry, the soil is very hard, making root growth difficult. Rooting depth of crops growing in sodic soils will be very shallow.

Figure 8. Soil with a natric horizon in North Dakota. (U.S. Department of Agriculture-Natural Resources Conservation Service photo)
Sodic Soil Nomenclature
The NRCS definition of the following sodic conditions are much more complete than presented here and can be found in USDA-NRCS, “Keys to Soil Taxonomy,” 2003 (p. 20), www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/nrcs142p2_051544.pdf.
- Natric soil — A soil with an argillic horizon, but with excess sodium with an SAR greater than 15 and/or pH greater than 8.2, possessing a natric horizon of distinct columns
- Glossic soil — A soil in which the natric horizon has degraded through time
- Leptic soil — A sodic soil with gypsum crystals present within 16 centimeters (cm) of the surface
- Typic soil — A sodic soil with properties between Glossic and Leptic
Although the definition of a natric soil above will be used by NRCS for the foreseeable future, research in North Dakota and in several other parts of the world indicate that dispersion and soil swelling become problems with an SAR greater than 5. For this reason for management purposes, sodic soils in North Dakota are defined as having an SAR greater than 5. The higher soil EC may serve to reduce the negative impact of high SAR.
Source of Sodium in North Dakota Soils
The major source of sodium in North Dakota soils is the shale bedrock that is present in the state. When glaciers moved across the state, some of the shale was ground up and incorporated in the ice. When the glaciers melted, the shale sediments remained as part of the glacial till material that makes up the sediments in soils north and east of the Missouri River.
South and west of the Missouri River, the glacial till from the previous glacial period (Laurentian) are mostly eroded away, exposing sodium-rich sediments from bedrock at least 65 million years old. The main exception to this sodium source is the area west of Grand Forks, where an artesian system brings up sodium chloride imbedded in an ancient seabed hundreds of feet below the valley surface. In this area, the salts are chloride-based, whereas in most of North Dakota, salts are sulfate-based.
Distribution of Sodic Soils in the Region
Figure 9 shows the relative distribution of sodic soils in North Dakota and the region. Sodic soils may be found nearly everywhere in the state, but in some areas, the sodic soils are small and may or may not be marked as “inclusions” in NRCS soil maps (Figure 10).
The major regions in the state with large areas of sodic soil are Burke and Divide counties in the northwest; the Bottineau and Langdon areas where the soil is shallow to shale bedrock; west-river, particularly in Slope, Bowman, Stark and Billings counties; Sioux County; and areas in Dickey and LaMoure counties near the James River.
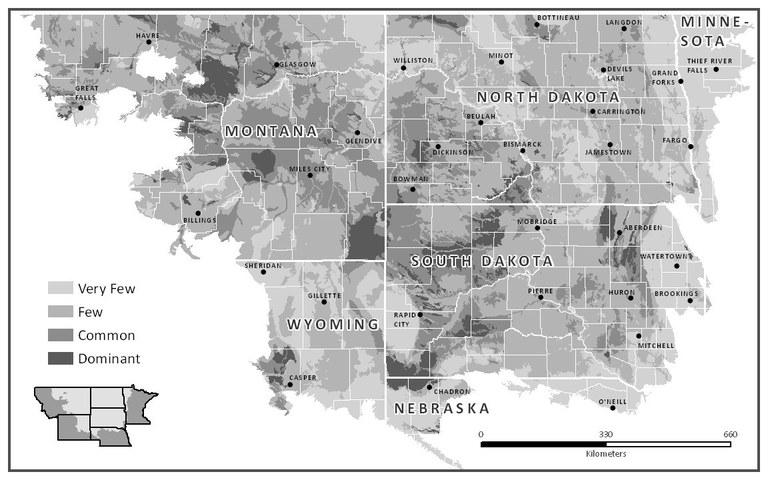
Figure 9. Relative distribution of sodic soils in North Dakota. (J. Brennan, USDA-NRCS, used with permission)
Figure 10. Sodic inclusions in a field near Wyndmere, N.D. (NDSU photo)
Remediation of Sodic Soils
Basic Requirements
The essential requirements for remediating sodic soils are:
- Adequate internal drainage
- A soluble calcium source
- Water in excess of crop requirements
The absence of any of the three essential requirements for remediation will result in the persistent presence of the sodic condition.
Steps in Preparation for Sodic Soil Remediation
Soil Sampling and Analysis
The soil sample should be taken to the depth of the intended tile drainage and the core divided into 1-foot increments. There may or may not be SAR differences to depth and only deeper soil sampling will provide the important information.
Variability of soil properties in sodic soils can be great (Figure 11), but understanding variability is important for proper management and expectations. Each depth should be analyzed for SAR, EC, pH, base exchange capacity, real cation exchange capacity (CEC) and calcium carbonate equivalence (CCE). As earlier stated, using the Na percent of the total base exchange capacity is a very good proxy for the more expensive SAR analysis (DeSutter et al., 2015). The following images may help explain the limitations of the most common CEC analysis.
The most common method of determining cation exchange capacity (CEC) in North Dakota is by the “summation” method (Figure 12). In this method, a solution of 1-molar ammonium acetate to provide excess ammonium to the anticipated base ions (calcium [Ca], magnesium [Mg], sodium [Na], potassium [K], hydrogen [H], aluminum [Al]) on the soil clay/organic matter surfaces is added. The ions associated with the clay/organic matter CEC sites are replaced by the ammonium ions, and the ions in the extracted solution are analyzed and quantified.
The summation method is a reasonable method for determining CEC in soil without measurable EC and free lime, but when soluble salts and/or free lime is present, the ions in the soluble salts are included in the extracted solution, along with those on the CEC sites, and some free lime is dissolved. The result is that when EC and/or free lime is present, the CEC is overestimated. Therefore, if EC is measurable and/or soil pH is greater than 7, particularly if the CCE is greater than zero, then the “real” CEC method illustrated in Figure 13 should be used.
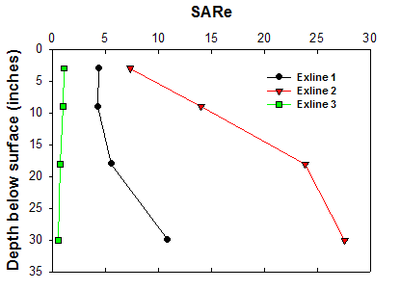
Figure 11. Changes in SAR with depth in a sodic Exline soil. Each sample was taken within a 10-acre patch of soil in the same field. (He and DeSutter, unpublished data, 2015)
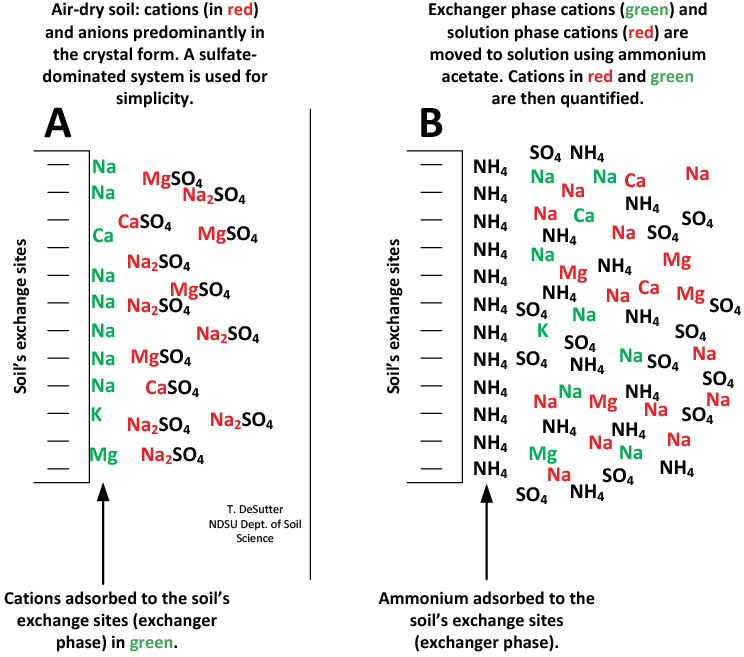
Figure 12. Soil with soluble salts (left) before ammonium acetate laboratory extraction and after (right). The CEC by summation method overestimates real CEC in soils with free lime and/or soluble salts.
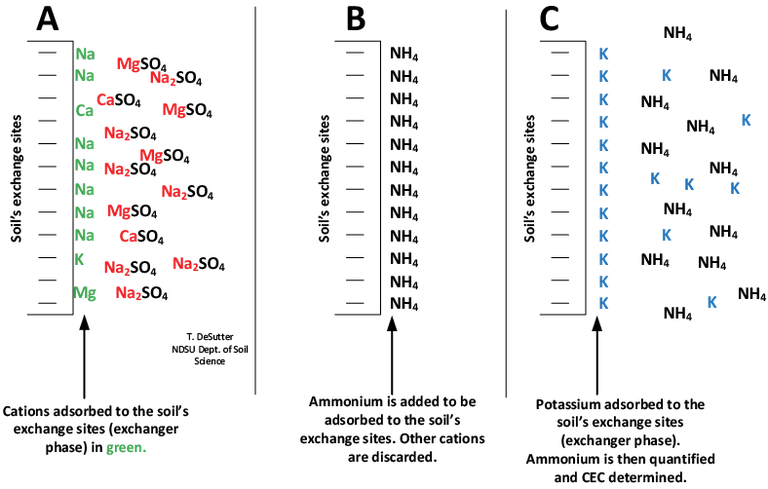
Figure 13. A method for “real” CEC determination. Ammonium acetate is added as extractant to the soil as in Figure 12; however, the extraction solution then is discarded. A potassium solution then is used to saturate the soil, resulting in removal of the ammonium ions from step B, then the ammonium is analyzed to provide the real CEC. This method provides real CEC but cannot be used to determine base saturation.
Drainage
Tile drainage is important, or the soil should be sufficiently permeable and have high enough topography (not in a depression) that water can move through the soil and away from the field. A landmark study from Illinois (Sharma et al., 1974) showed that gypsum is most effective if tilled to the depth of tile (3 feet in this study) and tiled. Mixing the soil without tile actually resulted in soil degradation and further loss of productivity, while a gypsum amendment, tile and mixing to the depth of tile resulted in corn yield increases and improved productivity (Figure 14).
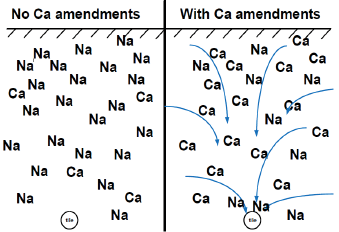
Figure 14. Illustration of the use of calcium amendments, primarily gypsum, to remediate sodic soils through tile drainage.
Calcium Amendment
The calcium amendment must be soluble. Table 1 shows several possible amendments and their solubility.
Calcium nitrate and calcium chloride are the most soluble calcium sources listed, and both are relatively expensive, compared with gypsum sources. Calcium chloride has the negative property of being a more active salt than its sulfate counterpart, which would be detrimental to crop productivity.
Many growers in the Red River Valley use sugarbeet waste lime for various purposes, and liming might be helpful for long-term sodicity control, but it would be too insoluble to help in the initial years. Many studies have shown the benefits of a soluble calcium amendment on sodic soil remediation (Rasmussen et al., 1972; Sharma et al., 1974; Oster and Frenkel, 1980; Webster and Nyborg, 1986; Amezeta et al., 2005; DeSutter and Cihacek, 2009).
Gypsum can come from a quarry, but it also may come from an industrial process or the end-product of coal/gas desulfurization (flue-gas gypsum). A recent study showed that flue gas gypsum is effective in remediation of sodic soils if the land is tiled (Table 2).
Table 1. Sources of calcium and solubility.
|
Source |
Solubility (g/L) |
|
Calcium nitrate |
1,212 |
|
Calcium chloride |
750 |
|
Calcium sulfate (gypsum) |
2.55 |
|
Calcium carbonate |
0.0062 |
Table 2. Results of flue-gas gypsum application to a sodic soil with and without drain tile, 2014-2017.
|
|
Treatment |
Na* |
EC |
|
|
Drainage |
Source |
Tons/acre |
% |
mmhos/cm |
|
Tiled |
Control |
0 |
4.9 |
1.0 |
|
|
Flue gas gypsum |
5 |
4.0 |
1.1 |
|
|
Flue gas gypsum |
15 |
3.1 |
1.4 |
|
|
Flue gas gypsum |
30 |
2.7 |
1.5 |
|
Nontiled |
Control |
0 |
9.9 |
2.3 |
|
|
Flue gas gypsum |
5 |
10.3 |
2.4 |
|
|
Flue gas gypsum |
15 |
8.4 |
2.3 |
|
|
Flue gas gypsum |
30 |
6.9 |
2.3 |
* Sampled at the 0- to 6-inch depth.
Importance of Calcium Amendment Mixing to Tile Structures or Depth of Rooting Restriction
Several studies have indicated the importance of mixing the soluble calcium amendment to the depth of root restriction or tile structures with sodium-affected soil (Rasmussen et al., 1972; Sharma et al., 1974; Webster and Nyborg, 1986). Without mixing, the calcium amendment cannot react with sodium-affected soil due to the impermeability of sodic layers. A deep-plowing implement is illustrated in Figure 15 for use in mixing amendments to 4 feet in depth.

Figure 15. A plow used to mix amendments to a depth of about 4 feet. (NDSU photo)
Remediation Equation for Gypsum Application
The amount of gypsum required for sodic soil remediation (gypsum requirement) is determined using the following formula:
GR = 0.86 (F) X (D) X (ρb) X (CEC) X (SARi – SARf) X (100/percent gypsum purity)
Where:
- 0.86 is derived for the mass of gypsum (CaSO4*2H2O) required to replace one Na+
- GR is the gypsum requirement in Mg per hectare (1 Mg/ha = 0.45 tons per acre)
- F is the Ca to Na exchange efficiency; 1.1 for SAR of 15 or greater, 1.3 for SAR of 5
- D is depth of soil to treat in meters (1 meter = about 3.3 feet)
- ρb is bulk density of soil, grams per cubic centimeter (g/cm3, usually between 1.1 and 1.6) (1g/cm3 = 1Mg/m3)
- CEC is the “real” CEC in mmol(c)/kg (1 cmol(c)/kg = 1 meq/100g = 10 mmol(c)/kg)
- SARi is the SAR (%Na) existing now
- SARf is the goal SAR (%Na)
- Gypsum is rarely pure, so purity is needed for correction to the mass of actual gypsum to be applied
Although calculating a gypsum requirement using this formula is possible, a much easier method is to use the NDSU Gypsum Requirement app for IPhones and Android phones (Figures 16 and 17).

Figure 16. Opening image of NDSU Gypsum Requirement app.
Figure 17. Input in NDSU Gypsum Requirement app.
Brine Spills
Produced water, commonly referred to as “brine,” is a byproduct from the extraction of oil and natural gas. Depending on the field, location and the age of the well, the oil:brine ratios in the Williston Basin (Bakken and Three Forks formations) range from 2:1 to 1:4, whereas in older formations where enhanced recovery is being used, this ratio can be 1:2 to as little as 1:100 (communications with various oil and gas professionals).
Although some exceptions occur, the chemistry of brine in the Williston Basin primarily is composed of NaCl, and brines have EC and SAR values exceeding 200 and 200 deciSiemens per meter (dS/m) (1dS/m = 1 mmoh/cm), respectively. Thus, when spills occur due to accidental (pipeline leaks, tank ruptures, vehicle accidents) and human-caused releases, Na and Cl can inundate the soil, which can greatly elevate EC, reduce biological activity and put Na on the cation exchange sites (Figure 18).
The most common remediation method is termed “dig and haul,” which essentially is removing the impacted soil, disposing of it in an approved landfill and replacing it with noncontaminated soil. Due the semi-arid environment in the Williston Basin, the use of Ca-amendments to reclaim impacted soils is a slow process and is dependent on the ability of the gypsum to solubilize Ca to replace Na on the exchange sites and Na to leach to deeper depths.
Because the Williston Basin only gets about 14 inches of precipitation per year and the potential evapotranspiration is between 45 inches and 50 inches per year, the ability of using only gypsum to reclaim these soils can be limited. The installation of tile drains beneath the brine-impacted zone, followed by flushing the soil with a highly soluble Ca-salt such as calcium nitrate and the collection and disposal of the leachate is another possible reclamation strategy.
As stated above, irrespective of the source of Na, the improvement or reclamation of sodium-induced soils requires a source of Ca, water of sufficient quality and leaching the Na below the crop-rooting zone.

Figure 18. Aftermath of brine spill in western North Dakota. (NDSU photo)
References
Amezketa, E., R. Aragues and R. Bazol. 2005. Efficiency of sulfuric acid, mined gypsum and two gypsum byproducts in soil crusting prevention and sodic soil reclamation. Agronomy Journal 97:983-989.
DeSutter, T.M., and L.J. Cihacek. 2009. Potential agricultural uses of flue gas desulfurization gypsum in the Northern Great Plains. Agronomy Journal 101:817-825.
DeSutter, T., D. Franzen, Y. He, A. Wick, B. Deutsch and J. Lee. 2015. Relating percent sodium to sodium adsorption ratio and its utility in the northern Great Plains. Soil Sci. Soc. Am. J. 79:1261-1264.
He, Y., T. DeSutter, F. Casey, D. Clay, D. Franzen and D. Steele. 2015. Field capacity water as influenced by Na and EC: Implications for subsurface drainage. Geoderma 245-246:83-88.
Oster, J.D., and H. Frenkel. 1980. The chemistry of the reclamation of sodic soils with gypsum and lime. Soil Science Society of America Journal 44:41-45.
Oster, J.D., I. Shainberg and I.P. Abrol. 1999. Reclamation of salt-affected soils. pp. 659-691. In Agricultural Drainage. Agronomy Monograph No. 38. R.W. Skaggs and J. van Schilfgaarde (eds) ASA-CSSA-SSSA, Madison, Wis.
Rasmussen, W.W., D.P. Moore and L.A. Alban. 1972. Improvement of a solonetzic (slick spot) soil by deep plowing, subsoiling and amendments. Soil Science Society of America Proceedings 36:137-142.
Sharma, A.K., J.B. Fehrenbacher and B.A. Jones Jr. 1974. Effect of gypsum, soil disturbance and tile spacing on the amelioration of Huey silt loam, a natric soil in Illinois. Soil Science Society of America Proceedings 46:113-117.
Webster, G.R., and M. Nyborg. 1986. Effects of tillage and amendments on yields and selected soil properties of two solonetzic soils. Canadian Journal of Soil Science 66:455-470.
For more information on this and other topics, see www.ndsu.edu/extension

