Increasing Food Safety on the Farm with Good Agricultural Practices (FN1920, May 2019)

Visit www.ag.ndsu.edu/fieldtofork for educational modules and more information about topics covered in this manual.
Purpose of this Manual
Small-farm operators may see the food safety certification process as a roadblock to getting their fresh produce into food service, institutional and retail markets. This manual assists specialty crop growers who have an interest in increasing food safety on their farm, documenting good agricultural practices, or becoming certified through the USDA’s Good Agricultural Practices and Good Handling Practices (GAP&GHP) audit verification program. Our goal is to help you understand the GAP&GHP audit cycle (before, during and after an audit) and identify potential benefits of becoming certified. The GAP portion of the audit pertains to on-farm production and harvesting practices, while the GHP portion of the audit covers the packing, storage and distribution of crops. This manual covers the GAP sections of the USDA audit verification program and does not cover the GHP sections.
You will benefit from this manual if you:
- Are a specialty crops grower.
- Would like to make your food safer for consumption.
- Want to learn more about GAP and GHP certification and how it might benefit you.
- Would like to market your products with buyers who require you to have certification.
- Want to know how to develop, document and implement a food safety plan.
This manual does not guarantee a successful USDA GAP&GHP audit. The success or failure of an audit is determined by how well you have identified and addressed the food safety risks around and within your farming operation. As we walk through the Audit Certification Checklist (in Section 5, page 33), we will point to the kinds of risks you should consider when tailoring a food safety plan for your farm. Throughout this guide, we identify how auditors use the checklist when inspecting farm operations. A better understanding of what auditors look for may help you pinpoint ways to become better prepared for the USDA GAP&GHP certification process.
This manual provides a general background on the USDA Agricultural Marketing Service GAP&GHP audit verification program. We provide content and examples to help you interpret the program’s requirements and demonstrate how audit items are scored so you may prepare when developing, implementing and maintaining your operation’s food safety program. This manual draws from content found in government agency publications:
- Food and Drug Administration (FDA) Guide to Minimize Microbial Food Safety Hazards for Fresh Fruit and Vegetables (dated October 1998)
- USDA Good Agricultural Practices Good Handling Practices Audit Verification Checklist (dated Sept. 18, 2014)
- USDA User’s Guide for GAP&GHP (dated April 2011)
- USDA Audit Instructions for GAP&GHP (dated November 2009)
Consider drafting your farm policies, standard operating procedures (SOPs) and corrective action plans while you work through this manual. Such documents will come in handy as you develop a food safety plan, whether your goal is to prepare for a USDA GAP&GHP certification audit or you simply want to implement the best practices possible for your farm operation.
We thank the following technical experts for their review of this manual:
Kim Butz
Local Produce Safety Coordinator
Carolina Farm Stewardship Association
P.O. Box 448
Pittsboro, NC 27312
Emily Griep, Ph.D.
Manager, Food Safety
United Fresh Produce Association
1901 Pennsylvania Ave. N.W. No. 1100
Washington, DC 20006
Background
Specialty crops are defined as “fruits and vegetables, tree nuts, dried fruits, horticulture and nursery crops (including floriculture).”2
The Agricultural Marketing Service1 (AMS) Specialty Crops (SC) Program helps specialty crop growers and handlers combine available resources to grow their respective industries and overcome marketing barriers. To do this, the AMS created strategic partnerships with state agencies and organizations for the benefit of growers, shippers, brokers, receivers, processors, retailers, restaurants, direct-to-consumer salespeople and the food service industry nationwide.
In the AMS Specialty Crops program, you will find a wide array of services, ranging from helping to market the quality of your products to ensuring that you experience fair trade in the produce industry. The USDA’s GAP&GHP audit verification program is a voluntary, market-access solution offered to the specialty crops industry.
In October 1998, the USDA and the U.S. Food and Drug Administration (FDA)3 issued guidance documents to the fresh fruit and vegetable industry for reducing microbial contamination of fresh produce meant for human consumption. The FDA’s “Guide to Minimize Microbial Safety Hazards for Fresh Fruits and Vegetables” (the FDA guide) included a number of recommendations for following Good Agricultural Practices (GAP) and Good Handling Practices (GHP). Soon after, many wholesale produce companies and food-service buyers of fresh fruits and vegetables began to require that their suppliers undergo third-party audits to verify supplier adherence to the FDA’s GAP and GHP recommendations.
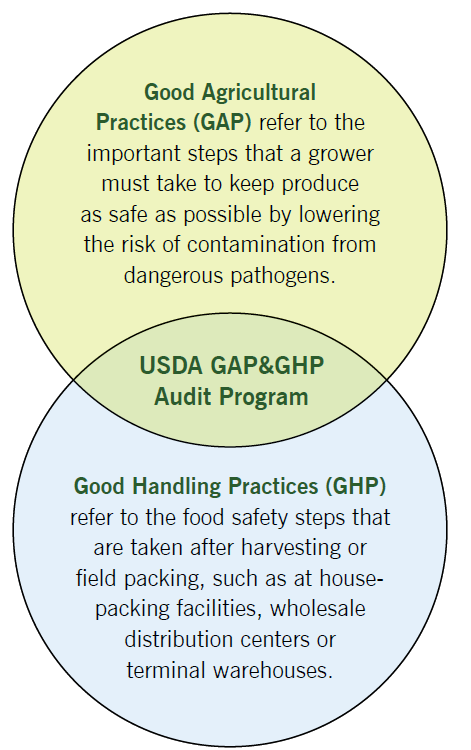
To address numerous requests from the fruit and vegetable industry, the USDA AMS, in cooperation with the Association of Fruit and Vegetable Inspection and Standardization Agencies (AFVISA), developed an audit-based program to verify suppliers’ conformance with the FDA guide. In August 2001, the USDA tested and approved a draft of the auditor checklist to satisfy the needs of growers and shippers. In January 2002, the USDA formally implemented the GAP&GHP audit verification program as a nationally recognized market-access tool that applies uniformly to the U.S. fresh produce industry.
The GAP&GHP program is a voluntary and user-fee funded program. Scheduled audits are intended to occur at least once per year to verify that a supplier’s crop(s) (e.g., fresh fruits and vegetables) have been produced, packed, handled and stored as safely as possible to minimize risks of microbial food safety hazards. The audit program does not guarantee that a supplier’s product is free from microbial contamination. Rather, the program verifies the extent that an audit participant has taken proactive measures to reduce risk of product contamination by adhering to generally recognized best practices. Continued observance of GAP&GHP best practices and responsibility of product safety remain with the operation in charge of producing and handling fresh products.
Adhering to generally recognized food safety best practices is not a new concept for many farming operations. However, the implication of fresh produce in major foodborne illness outbreaks in the U.S. has prompted buyers and suppliers to embrace a more proactive (rather than reactive) approach that protects the safety and well-being of consumers and minimizes the industry’s economic impact of food recalls stemming from foodborne illness outbreaks.
Market-Access Considerations
Many growers seek an audit to meet the requirements of particular buyers or a new market that requires third-party food safety certification. The USDA GAP&GHP audit verification program can be structured to meet your buyers’ requirements. Attaining certification may open new markets, such as schools, major retail grocery stores or wholesalers, for your products. Your current (or new) buyers may require that you pass the food safety audit before they will purchase your products.
If an existing or potential customer asks you for GAP certification, understand the customer’s needs and concerns. Which GAP audit sections are required to address those concerns? In the case of farm-to-school customers, is there a particular distributor already certified to serve the local school district? Will the school approve your products if delivered through that distributor? Depending on your operation and market-access needs, particular audit sections may be more or less cost-effective for you to perform. For example, Part 1 (Farm Review) and/or Part 2 (Field Harvest and Field Packing) may be more approachable for farms just getting started with food safety certification.
Tip for Audit Selection: Some buyers only will accept GAP certification from a private certifier. Identify which certifying agency your buyers prefer before investing time and resources into audit Services.
Audit Scopes
The USDA’s audit verification program is divided into seven sections, called scopes. As seen in Table 1, each scope covers specific aspects of the food supply chain. The USDA considers a GAP audit to consist of Parts 1 (Farm Review) and 2 (Field Harvesting and Field Packing Activities). A GHP audit consists of Parts 3 and 4 (for pre-farm gate operations) and Part 6 (for post-gate operations). Part 7 is an optional scope if you need verification of a food defense plan. With the exception of only conducting a Food Defense Audit (Part 7), the General Questions scope is required for every audit that takes place on your farm, regardless of buyer expectations. When planning your audit, identify which scopes to include.
Table 1.Scopes of GAP and GHP Audit Program
|
GAP |
GHP |
Audit Scopes |
|
X |
X |
General Questions |
|
X |
Part 1 – Farm Review |
|
|
X |
Part 2 – Field Harvesting and Field Packing Activities |
|
|
X |
Part 3 – House Packing Facility |
|
|
X |
Part 4 – Storage and Transportation |
|
|
N/A |
N/A |
Part 5 - (no longer used) |
|
X |
Part 6 – Wholesale Distribution Center/Terminal Warehouse |
|
|
Opt |
Opt |
Part 7 – Preventative Food Defense Procedures |
Onsite visits allow an auditor to observe and verify that you are implementing good agricultural and handling practices throughout your operation. For a Part 1 review, the auditor must visit and evaluate your operation while your crops are actively growing. For a Part 2 review, the auditor must observe your harvesting and packing activities. Depending on the size of your operation and the audit scope(s) requested, the auditor may need to make several trips to your location to conduct the initial audit. For example, the auditor may complete the farm review section on the first visit and the packing house section on a second visit.
Certification
For each GAP&GHP audit that you request (see page 19), you will have a unique audit verification program scoresheet. The auditor will use the scoresheet to summarize the evaluation of scopes under review. The summary will include the date and time that your audit began, as well as the date and time that the audit was completed. The auditor will note your pass-fail status for each scope and corresponding scores.
Audit Scopes Covered on the Same Day
Some audits can be performed on a single day’s visit. Of course, this is dependent on the type of scopes identified in the audit request and the complexity of the operation under review, including the number of days in operation and the commodities to be audited. If your GAP&GHP audit is completed without fail in a single day, the auditor will finalize the audit scoresheet by indicating “All Scopes Completed” and move the certification process forward by submitting the scoresheet to the appropriate reviewing official (e.g., supervisor). If approved at this level, the official will sign and forward your scoresheet to the USDA Branch’s Audit Program Coordinator (APC). In turn, the APC will generate your USDA certificate for the commodities covered in your audit.
Audit Scopes Covered Across Time Periods
If the auditor determines that additional visits to your location (e.g., announced and/or unannounced) are necessary to complete the entire audit process, the scoresheet and corresponding audit report will remain a work in progress. All detail collected by the auditor during subsequent visits will be added to the relevant scope sections of the scoresheet. As the scopes are completed during subsequent visits, the auditor will forward your scoresheet information to the appropriate reviewing official (e.g., supervisor) for a review and signature. This process will be repeated until all scopes have been covered across your audit period.
Once the entire GAP&GHP audit is completed without fail, the auditor will finalize your scoresheet by indicating “All Scopes Completed” and move the certification process forward following the same procedures described in the single-day audit. The auditor will submit your information to the appropriate reviewing official (e.g., supervisor). If approved at this level, the official will sign and forward your scoresheet to the USDA Branch’s Audit Program Coordinator (APC). In turn, the APC will generate your USDA certificate for the commodities covered in your audit.
For same-day and multiday audits, the certificate is specific to commodities covered in your requested audit and will be good for one year from the initial audit date. Your information pertaining to the completion of audits (for Parts 1 through 6 only) that meet USDA GAP&GHP criteria will be posted on the USDA website and will remain there for one year, unless you fail an unannounced verification visit in the meantime. (See Table 2, p. 6, for USDA excerpt of information posted.)
Audit Reports on Websites
The USDA Specialty Crops Inspection (SCI) Division can upload your USDA audit report to one or more commercial supply chain databases: ICIX, Azzule Systems or Food LogiQ. SCI charges one hour at the current audit rate ($108/hour) per each upload for each system that you choose.
You must pay the bill for the upload to maintain your certification listing on the USDA website.
Upload Options – Links to External Sites
- ICIX: https://www.icix.com
- Azzule Systems: http://www.azzule.com/
- Food LogiQ: https://www.foodlogiq.com/
General Auditor Policies
All USDA GAP&GHP auditors are either federal USDA AMS employees or state department of agriculture employees who have been specifically trained and licensed to conduct audits on behalf of the USDA AMS. Many of these licensed individuals are fruit and vegetable inspectors who grade produce on a daily basis. Individuals licensed by the USDA AMS use the GAP&GHP audit checklist to assess your operation. The USDA auditors are prohibited from performing a desk audit of another auditing firm’s results as verification of your conformance to the USDA GAP&GHP audit checklist. Additional information and the current USDA GAP&GHP checklist can be found at www.ams.usda.gov/gapghp.
USDA AMS policy states individuals licensed to perform GAP&GHP verification audits have no financial interest in the operations that they observe and products that they audit. This policy is in place to maintain the unbiased nature of the audit services and avoid any potential conflicts of interest. To remain impartial, the USDA auditors are prohibited from providing any form of consulting services to potential auditees. This means that the auditor is unable to offer recommendations specific to your operation on how you can conform to any questions on the GAP&GHP audit checklist. For example, the USDA auditor is not allowed to tell you how to write a food safety program for your operation or which actions you need to take to improve compliance with USDA program requirements and increase your audit score. When you have such questions, the auditors are obligated to direct your inquiries to food safety experts, such as Extension specialists. Contact the N.D. Department of Agriculture for assistance.
Table 2. Excerpt of Information Posted on USDA Website for Companies Meeting GAP&GHP Acceptance Criteria.
|
Company |
Address |
City, State |
Scope(s) of Audit Conducted |
Date Audit |
Commodities Covered by Audit |
|
Audit Type: USDA Good Agricultural Practices and Good Handling Practices Audit |
|||||
|
Commodity: Onions |
|||||
|
Location: North Dakota |
|||||
|
Benz Farm LLP |
519 5th Ave. S.W. |
Steele, N.D. |
Farm Review, Field Harvesting and Field Packing Activities, Storage and Transportation |
Oct. 2, 2018 |
Onions |
|
Commodity: Potatoes |
|||||
|
Location: North Dakota |
|||||
|
Bill Sheldon |
11028 51st St. N.W. |
Ray, N.D. |
Farm Review, Field Harvesting and Field Packing Activities, Storage and Transportation |
Aug. 30, 2018 |
Potatoes |
|
OC Schulz and Sons |
401 4th St. |
Crystal, N.D. |
House Packing Facility, Storage and Transportation |
Feb. 28, 2019 |
Potatoes |
Note: Report Name: G01 - By Audit Type Commodity Location and Company Name - Auditees that Meet Acceptance Criteria; Print date: 3/29/2019 9:18:57 AM. This excerpt shows variations in scopes and commodities for three of 44 North Dakota companies listed on the USDA website. For a complete and current list go to https://apps.ams.usda.gov/GAPGHP/reportG01.aspx.
Appeals, Complaints and Disputes
You have the ability to appeal, dispute or lodge a complaint regarding a USDA GAP&GHP audit or the individual licensed to perform the audit.
- Appeal: The USDA defines an appeal as a formal complaint contesting the results or findings of an audit brought before the Fresh Products Branch (the Branch) by applicants or other parties.
- Complaint: The USDA defines a complaint as discontent or unhappiness about a situation, interpretation or performance of an audit, auditor(s) and/or policy brought before the Branch by applicants or other parties.
- Dispute: The USDA defines a dispute as a disagreement or argument about a situation, interpretation or performance of an audit, auditor(s) and/or policy brought before the Branch by applicants or other parties.
Appeals, complaints and disputes must be submitted to the Branch within three calendar days from the date the audit service was performed.
Your formal request for appeal must be in writing and on your company letterhead. Your request must include the audit date, the location of the audit and the sections of the original audit being appealed, with clear identification of specific items under dispute.
Your appeal will be reviewed by the USDA Audit Review Board (ARB) authorized to sustain or reverse your appeal. The ARB will consist of a minimum of three members designated by the Branch chief. Members will be Branch headquarters staff (e.g., federal program managers) with good standing as audit program evaluators.
The ARB meets as needed to review audit appeals, and provides results of an appeal to all parties involved in the appeal process within 14 calendar days. Copies of your appeal and the ARB results will become a permanent part of your audit record.
Direct complaints regarding Branch policies and procedures to the Audit Management Section.
Complaints regarding an individual performing your audit should be directed to the auditor’s supervisor.
The Branch retains records of all appeals, complaints, disputes and subsequent actions taken that pertain to GAP&GHP audits. Additionally, the branch reviews and evaluates the effectiveness of all appeals, complaints, disputes and subsequent actions on a regular basis.
USDA
1400 Independence Ave. S.W.
Stop 0247
Washington, DC 20250-0247
Audit Costs
You will incur expenses each time the auditor needs to prepare for an audit and observe your operation for certification purposes. Your audit charges will include an administration fee. You will be charged for the auditor’s time, including time spent reviewing your food safety manual (in preparation for your audit) and travel time to and from your farm and actual time spent at your farm (to observe your operation). The expenses for follow-up visits to your farm will be charged in a similar manner to cover the auditor’s administration fees, prep time, travel time and time on the farm to conduct the follow-up inspection. It is possible that the auditor’s second unannounced visit will be smaller in scope, where the focus is on field operations and a review of your logs and records. As evidenced in Table 3, the auditor’s time has a significant impact on your bill.
Table 3. Example of May 2019 audit estimates.
|
Expense Type |
Time |
Charge |
|
Administration fees |
$ 50 |
|
|
Preparation time |
1 hour |
$ 108 |
|
Travel time to farm |
3 hours |
$ 324 |
|
Audit day time |
2.5 hours |
$ 270 |
|
Travel time from farm |
3 hours |
$ 324 |
|
Paperwork |
1 hour |
$ 108 |
|
Total Cost |
$ 1,184 |
Consider organizing your food safety manual, including SOPs and supporting documents, in the order of the GAP audit checklist. During the audit, the inspector will follow the USDA checklist. If your documents are organized in the same order, it may help your audit go more smoothly and quickly, and, in turn, save you money since audits are charged by the hour.
You are the best advocate for your operation. Prepare well for the audit and help the auditor realize that only one inspection may be necessary. Show as many crop production examples as possible during a single audit visit and help the auditor understand your processes. For example, hand harvesting tomatoes may satisfy the auditor’s need to see the harvest of other vegetables that are not grown in contact with soils that you also hand harvest.
To save money, try to coordinate your audit when others in the area may need an inspection. Perhaps you can request your audit for the same or subsequent day and share in the cost of the auditor’s travel time. To find others interested in coordinating audits, check with neighbors, ask operators at the farmers market, ask buyers or seek out university Extension agents. An auditor will divide the travel costs between farms if multiple (and nearby) sites can be audited during a single trip to your area.
Cost Savings Tips:
- Follow the USDA GAP&GHP audit checklist to organize your food safety manual and supporting documents to help your audit go smoothing and quickly.
- Prepare well for the audit and help the auditor see why only one inspection may be necessary.
- Try to coordinate audit dates with others in your area to share in auditor travel fees.
Your Food Safety Program
Establishing and implementing a food safety program is highly recommended for any operation responsible for producing and handling fresh fruits and vegetables, not just for those wishing to participate in the USDA GAP&GHP audit verification program. During a GAP&GHP audit, you will be asked to explain and demonstrate how your operation complies with industry best practices and more specifically the FDA’s recommendations for minimizing microbial safety hazards for fresh fruits and vegetables. Before you can participate in the USDA GAP&GHP audit verification program, you are required to develop and implement a documented food safety program that applies specifically to your operation.
Food Safety Manual
Normally, a food safety program takes the form of a written manual that (a) details all aspects of the growing and handling processes; (b) identifies potential sources of risk of microbial, physical and chemical hazards; (c) outlines specific, measureable steps necessary to reduce the risk of product contamination; and (d) describes steps that you have taken when product contamination has been suspected or realized.
Consider three main steps as you start to assemble your food safety manual.
First, write a mission or vision statement that captures your operation’s commitment to food safety, food quality, food sanitation, and worker health and hygiene. Write your statement in a brief, general yet thorough manner to adequately describe the extent that you and your employees embrace your food safety program. Place the statement at the front of your manual. An example of a mission statement might be:
Management and employees of Specialty Crops Incorporated are committed to producing and marketing the safest products possible through the implementation of good agricultural and handling practices that focus on the principles of food safety and quality.
After writing your mission statement, write a description of your farming operation. Describe the physical location of your operation, all the acres that you farm (whether owned, leased, rented, contracted or consigned), where all your production areas are located (including primary and secondary locations) and the various crops that you grow. If you have a packing house facility, include a floor plan to indicate the flow of product (e.g., entering and leaving the storage areas), areas where products may be commingled or culled, employee breakrooms, restrooms, offices, and where pest control devices and water lines are located. Include maps of your farm fields to show surrounding irrigation sources, hydrants and other details. Sketch out a corresponding map of your farm and insert it after your written descriptions. For farm map examples, see Figure 2 (p.12) and Figure 3 (p.13)
Second, create a table of contents that corresponds to the audit scopes that will pertain to your operation. (Refer to the audit scopes in Table 1, p. 4.)
Third, write in detail how each of the GAP&GHP audit scopes pertains to your operation. As you describe a scope, provide detail about how your operation is complying with the requirements of that audit scope. Insert copies of the related standard operating procedures (SOPs) and include examples of corresponding documents and forms that pertain to the scope you are writing about.
The key to developing a well-written plan is to include applicable documentation that will prove to an auditor that you have an established food safety program in place. Your written manual also may contain information or references pertaining to management reviews of your food safety program or any self-audits that have been conducted to test the program’s effectiveness.
Food Safety Officer
If your operation is serious about following a food safety program, designate an individual with sufficient food safety knowledge to ensure that the program is being followed. Whether the designated individual (e.g., food safety officer) is you, a cooperator or some other person on staff, you must name the individual in your food safety manual (e.g., use an organizational chart or some similar documentation). In this manual, we use the term “food safety officer,” although your organization may use a different title (e.g., manager, supervisor, etc.).
The food safety officer needs to comprehend all elements of the operation’s food safety practices, right down to the details provided in the food safety manual. You and your food safety officer need to be readily available to answer auditor questions whenever your operation undergoes any form of the USDA GAP&GHP audit. In preparation for an audit, it is vital that the two of you (a) determine what to document in your food safety manual (based on the GAP audit checklist); (b) maintain current and accurate records that reflect your farm’s adherence to policies and procedures stipulated in the manual; and (c) document any corrective actions that have been taken to adjust your food safety plan.
Establishing, implementing and maintaining an effective food safety plan involves the evaluation
of many processes. Find links to numerous
resources on the USDA GAP&GHP website
www.ams.usda.gov/gapghp. State and federal agencies, university and Extension programs, and trade and commodity associations offer additional guidance for food safety planning.
Tips for Building your Food Safety Program
Be sure your food safety manual includes:
- Your food safety mission statement
- The name of your designated food safety
officer- Records to show any completed GAP&GHP trainings
- A map that accurately represents your farm and shows all growing areas.(See examples, Figure 2, Figure 3)
- Legal descriptions (e.g., county-issued parcel number)
- Locations of all production areas (also secondary parcels)
- Total number of acres farmed
- Locations of all packing facilities
- Packing facility floor plans (show product flow, storage areas, cull areas, employee breakrooms and bathrooms, offices, etc.)
- Documents pertaining to commingled product (from different growers) that occurs at your farm
- Food safety practices that are relevant to your farm and can be fully implemented
- Written SOPs that define policies and procedures you and your staff follow to carry out the plan
- Written logs of your farm’s activities, including training practices, water test results, mock recalls, etc.
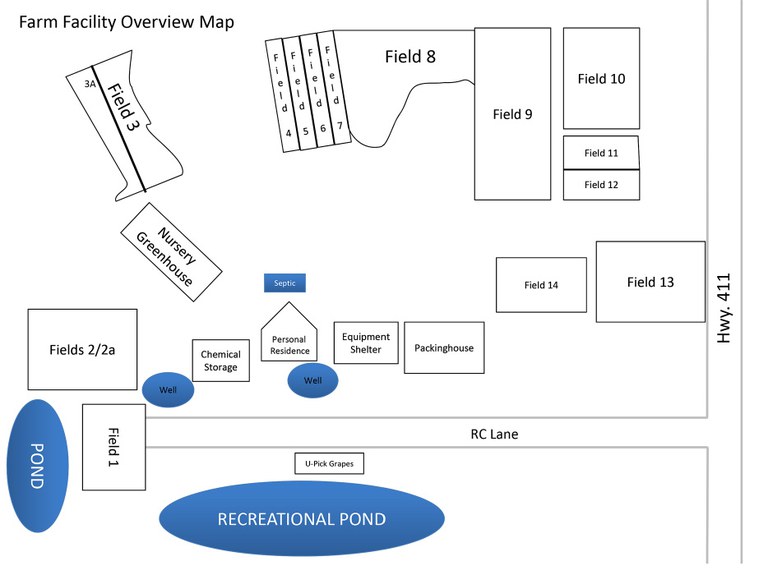
- Figure 2. Farm Map Example 1. Image used with permission from North Carolina Farm Stewardship Association. www.carolinafarmsteadstewards.org
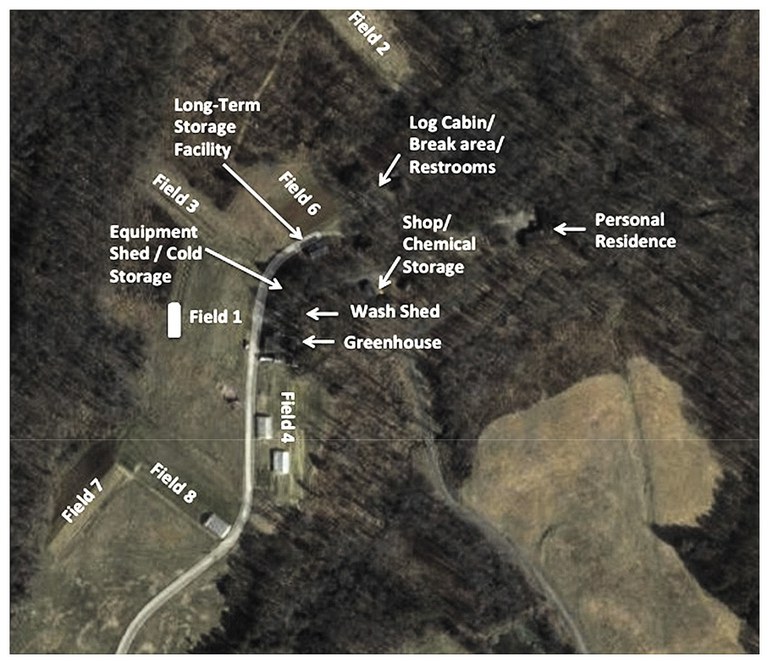
- Figure 3. Farm Map Example 2. Image used with permission from North Carolina Farm Stewardship Association. www.carolinafarmstewards.org
Record Keeping
Your operation’s records are key components of your food safety plan. Since the auditor is not at your operation on a daily basis, your records provide evidence that you have policies and procedures in place and that you have completed certain tasks throughout the year to maintain a consistent level of conformity to your food safety program. Understand the difference between the documentation requirements so that you will be prepared when the auditor asks to review your items during the audit. If you plan to participate in the USDA verification program, the GAP&GHP audit checklist defines three types of documentation that are required of your operation.
As seen in Figure 4, the documentation requirements are identified in the far right “Doc” column of the GAP&GHP audit checklist. A letter “P” in the checklist “Doc” column indicates that a policy/standard operating procedure (SOP) must be established and documented in your food safety plan to explain what you will do to conform to a particular audit question requirement. A letter “R” in the “Doc” column indicates that you must keep records to prove that an action or process has been completed. Records may take the form of schedules, work logs, checklists, service records, billing forms, service contracts, water test results, etc. A record is your written verification that shows when, why, how and by whom a specific task or procedure has been done.
Figure 4. Excerpt from the USDA GAP&GHP Audit Checklist for Worker Health and Hygiene.
|
Worker Health and Hygiene Questions |
||||||
|
Questions |
Points |
Yes |
NO |
N/A |
Doc |
|
|
G-3 |
Potable water is available to all workers. |
10 |
R |
|||
|
G-4 |
All employees and all visitors to the location are required to follow proper sanitation and hygiene practices. |
10 |
P |
|||
|
G-5 |
Training on proper sanitation and hygiene practices |
15 |
D |
|||
|
G-6 |
Employees and visitors are following good hygiene/ |
15 |
||||
|
G-7 |
Employees who handle or package produce are washing |
15 |
||||
|
Note: The abbreviations (D,P,R) identify your documentation requirements for an audit |
||||||
A letter “D” in the “Doc” column means that you must keep a combination of SOPs outlining your company policies as well as records indicating that a particular action was taken. At minimum, you must keep and coordinate the relevant documentation for the questions identified with a “P,” “R” or “D” in the “Doc” column, although you may find it important (if not necessary) to keep additional documentation beyond what the USDA requires. The bottom line is that you must maintain sufficient records to show adherence to your food safety program as well as to USDA GAP&GHP requirements, and local, state and federal regulations. (See the hypothetical example of record keeping below)
Hypothetical Example of On-Farm Record Keeping
All farms requesting an audit of "Field Harvest and Packing Activities" must have a written policy (in the SOPs) that documents the preharvest assessment and must maintain records to prove that the assessment has been completed as specified. The assessment should include a review for evidence of crop damage or intrusion caused by domesticated or wild animals.
Scenario
- The Problem: The preharvest assessment ( or other field review) for your farm indicates a threat to your tomato and strawberry crops from excessive animal intrusions. The deer have been eating the leaves and fruit of the tomato plants. You have notices oblong, pellet-like droppings and deer tracks in the tomato production area. Your tomato fields are adjacent to you strawberry production areas, where you have found evidence of racoon droppings. Fencing the fields is not an option because deer can jump and racoons can climb.
- The Solution: To deter the deer, you mount solar-powered, motion-activated lights on movable stands and place them around the tomato production area, along with some white flags about the size and shape of a deer tail (10 inches long, 5 inches wide). When the deer see movement, your hope is that they will get spooked and flee. To deter the racoons, you place solar-powered predator deterrent lights throughout the strawberry field as well. The lights come on when night falls and stay on until daybreak. The lights flash and mimic the eyes of other animals, which should trigger the flight response in the racoons. You plan to monitor the fields regularly and move the deterrent devices around to maintain the element of surprise for the animal intruders.
For GAP's record-keeping (R) requirements, you must keep a written log that documents the placement of the flags and lights in the fields, tracks your ongoing monitoring efforts, and details any further crop damage and intrusions. The auditor will review your log during your audit. Your records (e.g., crop maintenance reports or field logs) should clearly document: (a) that your trained staff is monitoring the implemented solution for effectiveness, (b) any changes you or your staff have made based on observations, and (c) any corrective actions taken when the best practice strategies have proven ineffective. For example, your farm workers should be trained to: (a) make sure the white flags and solar lights are in place and not blown over by a storm; (b) monitor the crops for animal droppings and indications of eaten plants and fruit; (c) put red flags at every site location that displays evidence of intrusion (including animal droppings). In turn, all harvest crews should be trained to avoid harvesting any products within a 5-food diameter of any red flag. Your written log also should include all efforts to train the staff for this issue.
Test Your Food Safety Program
Although not a requirement of the GAP&GHP audit verification program, your operation should conduct a self-audit prior to scheduling your USDA audit. You can utilize the USDA GAP&GHP audit checklist to perform an internal audit of your operation. A self-audit provides the opportunity to identify challenge areas, make improvements, modify SOPs if necessary and take corrective actions before you undergo the USDA audit.
If you plan to participate in the USDA GAP&GHP audit program, there are advantages for testing and evaluating your food safety program as close as possible to the beginning of your growing season. You will have more time to accumulate documents and records that will be needed for the auditor to review while he or she performs your audit. You may run into a situation where you need to change policies and procedures to improve the efficacy of your food safety program (e.g., need to implement a procedure to reduce wild animal intrusions in a new crop production area). Once the growing season is underway, you may have limited time and resources to develop and fully implement a viable food safety program for your operation. Many growers take advantage of the winter season while the fields are less busy and start planning for their first-time audit to take place during the upcoming harvest.
Create and complete a checklist (see Figure 6) to determine your operation’s readiness for scheduling an audit. File the checklist in your food safety manual, next to your audit request form.
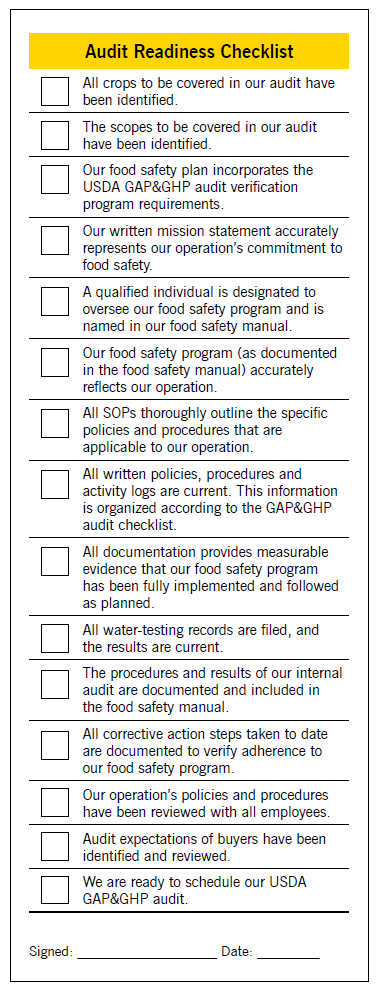
- Figure 6. Audit Readiness Checklist. Figure developed by Kimberly Beauchamp, March 2019.
Required USDA Forms
Vendor Account Agreement
Prior to scheduling an audit, set up (or update) your vendor account with the USDA Specialty Crops Inspection (SCI) Division. The USDA will not bill you for services until after certification or audit documentation is completed. However, failure to establish an account or pay the bill may result in the cancellation of your USDA audit certification. You may submit the completed form (SC-430) to the USDA SCI Division using any of the following options:
- Email: SCRimbursement@ams.usda.gov
- Fax: 866-230-9168
- Mail: USDA, AMS, SCI, ASB
1400 Independence Ave. S.W.
Stop 0247, Room 0707-S
Washington, DC 20250-0247
Form SC-430: https://www.ams.usda.gov/resources/sc430
Audit Participation Agreement
You (or your food safety officer) must sign a participation agreement (Form SC-651) and provide the form to the auditor prior to the start of your USDA GAP&GHP audit. This contractual agreement outlines your requirements for participating in the USDA verification program. The form also clarifies the audit services you will receive.
As an auditee, your signature indicates that you agree to conform to laws, regulations and statutes that are applicable to the USDA GAP&GHP audit program and that you will submit your audit request at least two weeks prior to the end of your growing/harvesting/packing season. You also agree to maintain appropriate records of your operation and provide these to the auditor for review. Further, you must grant the auditor access to facilities at your location as necessary to appropriately perform the audit of requested audit scopes. Finally, you must agree to pay your bill in a timely fashion.
The auditor will sign the form on behalf of the USDA AMS. The performed audit will reflect an objective third-party verification that is based on internationally recognized audit principles. The auditor will provide an opening interview to discuss the audit’s agenda with you and an exit interview to share findings. During the exit interview, the auditor will provide you with hard-copy records of the audit report and deficiency notices (if any were found). The AMS agrees to respect the confidential nature of your operation when copying records as evidence of your operation’s practices. Any documents submitted to the AMS by the auditor will be considered voluntarily provided as your participation in the GAP&GHP verification program. The AMS agrees to issue you a certificate when all scopes have been successfully passed and agrees to publicly post and share your audit results only if you grant permission to do so.
The signatures on the form indicate a mutual agreement between you and the AMS. If you are eligible for USDA GAP&GHP certification, the issued certificate will cover a 12-month period that begins with your initial audit date. Your contractual agreement with the AMS will end (i.e., rescind the related benefits) if the auditor discovers that you no longer meet the requirements of the audit program. To reinstate the agreement (and AMS perks), the auditor will need to find evidence that you have effectively corrected all noted deficiencies. Form SC-651 is at
https://www.ams.usda.gov/resources/sc651.
Audit Request
To attain and maintain GAP&GHP certification, the USDA audits should be performed for your operation at a minimum of once per year. Your certification from a particular audit automatically will expire after a 12-month period. It is your responsibility to schedule audits for your operation. The USDA will not contact you to schedule annual announced audits.
Audit Request Form SC-237A
To request an audit, complete and submit the USDA Request for Audit Services Form (SC-237A). This form ensures that you receive the correct audit services for your operation and that the correct information will be recorded on your GAP&GHP certificate once you pass the audit. This form requires:
- Anticipated audit date(s)
- Farm contact information
- Farm /facility information (location, acres)
- List of commodities to be covered by the audit
- Type of audit services requested (e.g., USDA GAP audit) and which scopes you are requesting the audit to cover.
When submitting Form SC-237A, include multiple dates (up to six) that reflect harvest times right before scheduled deliveries (e.g., day before the farmers market). Also, specify the time of day you plan to harvest (e.g., before 9 a.m., after 3 p.m., etc.) to help the auditor plan to be on the site to observe you perform the activities.
To request an audit in North Dakota, contact: N.D. State Seed Department, 701-231-5400
When contacting the N.D. State Seed Department, be prepared with a list of anticipated audit dates that your operation will be available. All audit services are assigned on a first-come, first-served basis. Once you are assigned an initial audit date, the auditor will contact you to make arrangements. You will be provided with confirmation of your audit date and time, the audit agenda and an estimated cost for performing the services.
For each audit request (Form SC-237A) that you submit, you must provide a copy of your food safety manual and a list of the crops (including acreage) that you want the audit review to cover (see Table 4 for an example). Audit timing is critical for maximizing the value of your investment in certification. The key is to schedule your audits to get the most out of your 12-month GAP&GHP certification coverage for declared crops.
Example: Diversified Farm
Table 4. Example of Multiple-Crop Audit List
|
Example Crops |
Acreage |
|
Herbs |
1.00 |
|
Strawberries |
1.00 |
|
Tomatoes |
1.00 |
|
Carrots |
1.00 |
|
Summer squash |
1.00 |
|
Winter squash |
1.00 |
|
Brussels sprouts |
1.00 |
|
Pumpkins |
1.00 |
|
Total Acres |
8.00 |
On April 1, Dakota Farms submits a request for audit (Form SC-237A), a copy of its food safety manual and a declaration of eight crops. The initial audit visit is scheduled for June 30 and will cover the General Questions and Part 1 (Farm Review) for the entire farming operation. Additionally, the auditor will perform Part 2 (Field Harvesting and Field Packing Activities) for the herbs and strawberries that are scheduled for harvest. Because the operation’s production period is greater than 90 days, the auditor explains that there will be two unannounced verification visits during the remaining growing season. At later dates, the auditor returns (unannounced) to observe field harvesting and packing activities for the tomatoes, carrots, summer squash, winter squash, Brussels sprouts and pumpkins.
Let’s consider several audit-scheduling options to maximize the coverage.
If you want to certify a single crop with a short production season (e.g., strawberries), you could complete a scheduled audit at the beginning of the production cycle and sell GAP-certified products throughout the season. If you are a diversified farmer, you could schedule separate GAP&GHP audits for each commodity grown on your farm or schedule a single audit to cover a group of commodities.
However, three conditions apply if you choose to include multiple commodities in a single GAP&GHP audit review.
First, your multicommodity audit request must declare all commodities (e.g., strawberries, onions) that you would like to have covered in the audit review, and your manual must address the various food safety risks associated with the crops being audited. You cannot expand a requested audit at a later time to cover additional commodities. For example, if you are a berry grower and hand pick strawberries but mechanically harvest blueberries, your food safety plan must address potential food safety risks and corrective actions associated with both types of harvesting processes.
Second, the auditor must have the opportunity to observe all of the declared crops as you grow and harvest them. This requirement pertains to the initial announced audit review and any subsequent unannounced verification visits (see p. 27) that take place at your location. For example, if you schedule your audit for Aug. 1, any crops that will have been completely harvested before Aug. 1 cannot be listed on your audit request form. While the auditor may not actually observe the harvest of every declared commodity, the auditor needs to be able to observe the types of harvesting activities for certification purposes.
Third, your operation is required to participate in the appropriate number of unannounced verification visits, as determined by the length of your growing season and the declared commodities under review.
Tip for Submitting Form SC-237A:
- Send your Request for Audit form at least two to three weeks in advance of your desired inspection date.
- Include a copy of your food safety manual.
- If you plan to certify multiple crops on a single audit, schedule your initial review to take place: (a) between two production seasons, (b) when the auditor will observe the greatest variety of crops being harvested, or (c) when the auditor will be able to observe your harvesting and/or packing processes.
Audit Cycle
The GAP&GHP audit cycle comprises key phases that will require the auditor’s time, including offsite preparation (planning the audit) and onsite audit activities: opening meeting, actual audit, scope assessment and exit meeting.
Auditor’s Offsite Preparation
The offsite preliminary desk review helps the auditor gather basic information that is necessary to complete your onsite audit. The auditor will prepare for the upcoming visit by reviewing the GAP&GHP specifications, looking through your written food safety manual and identifying specific areas at your location that need to be audited. The auditor needs to form a thorough understanding of your operation to plan the direction of your upcoming audit. The planning criteria is driven by the extent that you have written and implemented a food safety program. The auditor will use the desk review to become more familiar with your operation’s food safety plan. The auditor also will take into consideration any current problems or corrective actions that may be on file and pertain to your operation.
The USDA GAP&GHP audit checklist serves as a systematic path that the auditor will follow when evaluating your operation. The auditor will use this checklist to form opinions and judgments of your operation’s conformity to GAP&GHP procedures, specifications and regulations. The preparation phase can take up to several hours of the auditor’s time, depending on the complexity of your scheduled audit, the organization and completeness of your food safety manual, and the size of your farming operation.
As you participate in yearly GAP&GHP audits, you will build an audit history. When planning for upcoming visits, auditors likely will consider any information recorded from previous announced and unannounced visits to your farm. You can expect problem areas that surfaced during previous audits at your location will be evaluated during upcoming visits to ensure that corrective actions previously taken remain effective in preventing reoccurrence.
As auditors become familiar with your operation, they will need less time to prepare for ongoing visits to your location. Your audit history is formed and the audit cycle continues with each new audit. Your audit history helps expedite an auditor’s planning process.
Onsite Audit
For your audit to be successful, the auditor must visit and observe your farm/facility while it is in operation. As seen in Table 5, the auditor will follow a step-by-step process each time he or she visits your location. Generally, you should plan for an onsite audit to last two to five hours. Of course, the length of time will depend upon the size, scope and type of audit being performed, as well as the complexity of your operation. For example, an audit for a small owner-operated farm would take considerably less time compared to an audit for a larger diversified farm.
Table 5. Typical Audit Day Process
|
Audit Day |
|
|
1. Opening Meeting |
|
|
2. Audit is Conducted |
|
|
3. Auditor Assesses the Scopes |
|
|
4. Exit Meeting |
Opening Meeting
Upon arrival, the auditor will make his or her presence known and immediately meet with your food safety officer and you. During this opening meeting, the auditor will clarify the purpose of the visit and verify that you have signed a contract to cover the intended audit services. If you have not yet signed the Participation Agreement Form (SC-651), the auditor will collect your signature during the opening meeting. Once your signature is in place, the auditor will explain the audit agenda, confirm the audit scope(s) that you have requested and communicate how the audit will proceed. The auditor will refer to the GAP&GHP checklist and ask for pertinent records and documentation that need to be reviewed. During the opening meeting, you and your food safety officer will be able to ask questions about the audit process. The opening meeting serves to clarify what the audit process entails so you and your food safety officer know what to expect throughout the day. The duration of the opening meeting is largely dependent on the complexity of your audit scopes and your familiarity with the GAP&GHP audit verification program.
Conducting the Audit
The auditor will follow the GAP&GHP audit checklist and keep notes while reviewing your documents and records, interviewing employees, examining products, and observing the processes and procedures of your operation. The checklist and notes will help the auditor form a determination of how well your operation complies with the written food safety plan and the USDA GAP&GHP program requirements.
In some instances, an auditor may need to review additional records to verify your conformity to a particular question, even though the checklist item does not indicate that documentation is required. As noted earlier (see p. 16 of this manual), the majority of the checklist questions are designated with the letter “P,” “R” or “D” to remind the auditor when to review policies, records or a combination of both. The coded reminders assist the auditor in verifying the extent that your documentation accurately represents your operation’s food safety program and that your policies, procedures and food safety manual conform to the USDA GAP&GHP requirements and local, state and federal regulations.
Process verification is an important component of conducting an audit. The auditor will ask questions of your employees and contracted staff during the observation process. The practices and actions of your employees and contracted staff will be observed for compliance with standard operating procedures and food safety requirements. The auditor’s observations build upon the items listed on the GAP&GHP checklist. You can expect the auditor to focus on areas of importance such as the worksite, rules that employees must follow, safety regulations, etc. As discoveries are made, the auditor will document observations and findings. When nonconformity is observed, the auditor will ask the person(s) responsible in the area of concern to review and confirm the finding. If your policy is to require special training, education, experience or certification for certain employees or contracted staff, the auditor will examine and verify the status of requirements met by all applicable individuals.
Assessing the Scopes
Once observations around your operation are complete, the auditor will need a private area to review his or her notes and findings, tally the audit scores for each scope reviewed, determine the severity of nonconformities (if any exist), make necessary telephone calls to USDA supervisors, identify relevant documentation, write the audit report and make copies of the report, including all supporting documentation.
The product of the auditor’s review is a written report of your operation. A number of important items constitute the audit report: the completed checklist, a scoresheet and the corrective action report (CAR). The auditor will initiate a CAR if he or she has observed any nonconformities or “automatic unsatisfactory conditions” during the review of your operation.
When an error is discovered and the documentation contradicts the checklist question requirement(s), the auditor will score that question as lacking conformity to your food safety program. The auditor will photocopy any documents or records that are discovered to be inaccurate and/or reflect deviations from your operation’s SOPs. The photocopies of such documents will become official records of your audit report. However, when such documents contain your personally identifiable information, the auditor will include written descriptions of the operational deviations in the audit report instead of photocopies of the documentation. Overall, the CAR will include the auditor’s written descriptions of any observed practice(s) or procedure(s) that led to your audit failure. Such detail will include the time, location and specific checklist question(s) or item(s) that the auditor observed and noted as lacking conformity to food safety standards recognized by the USDA and industry.
Exit Meeting
Generally, the last phase of the audit day is the exit meeting that takes place between the auditor, your food safety officer and you. During this meeting, the auditor will review the findings and discuss observations. The duration of the exit meeting will depend on the size of your operation, the audit scope(s) covered and amount of detail in the audit report. Within your audit report, you will find written explanations for all checklist questions that the auditor marked with “No” or “N/A.” Keep in mind that the primary purpose of conducting the audit is to: (a) verify your operation’s compliance with the USDA GAP and GHP audit checklist items, (b) verify the implementation of your written food safety plan and (c) highlight any corrective actions that you will need to take in response to nonconformities discovered during the audit.
You will be provided a completed copy of the audit report plus any supporting documents and supplemental records that have been attached to the report. At this time, you will be have opportunity to ask questions about any aspect of the audit and address the checklist items that the auditor marked “No” or “N/A,” including the corresponding explanations for such responses. Information that is discussed during the exit meeting may have a bearing on the nonconformity report that the auditor has prepared. Before leaving, the auditor will inform you of the number of required follow-up and unannounced surveillance visits that you can expect.
Nonconformities/Corrective Actions
The auditor is obligated to file a corrective action report (CAR) for any GAP&GHP audit that fails due to nonconformities from a specific “automatic unsatisfactory” condition or when a particular audit scope fails to meet the required minimum program requirements (e.g., a passing score). The auditor must submit the CAR prior to conducting a follow-up audit at your location. A follow-up audit can never be performed on the same day as your initial audit visit because the auditor must allow adequate time for you to fully implement long-term corrective actions and address problems identified in the CAR.
If you wish to continue in the GAP&GHP verification program, you must develop a plan to resolve all nonconformities listed on the CAR. You will typically complete two steps to address the CAR requirements: (a) short-term corrective action(s) and (b) long-term corrective action(s) often called root-cause analysis. Short-term corrective actions are steps that you will take immediately to address deficiencies noted in the CAR. Long-term corrective actions require more time because you need to find the root cause of the deficiency and determine if the problem occurs frequently or requires a long-term investment in resources to prevent recurrence. Typically, the implementation of long-term corrective actions will require changes in your standard operating procedures or policies that comprise your food safety program.
You are required to write a corrective action response plan that includes a list of short-term and long-term actions to be taken to effectively address the problem(s). You must submit your written response plan to the auditor for review and approval. The auditor will determine the efficacy of your short-term solution(s) and expect your root-cause analysis to include steps that will prevent recurrence. The auditor will look for your short-term corrective action plan to review (a) details of the operational failure(s), (b) immediate solutions to address the problem(s), (c) identification of an individual responsible for verifying that corrective actions are taken, and (d) the extent that all corrective action(s) conform to the policies and SOPs of your food safety program. (See below for a hypothetical example.)
Hypothetical Example of Nonconformities and Corrective Actions
Scenario
The auditor checked all four of your farm’s restroom facilities throughout the audit day and consistently found used paper towels spilling out of the trash receptacles and piles of used paper towels and other trash items scattered across the floors. The auditor confirmed that this unsanitary situation extended beyond a single restroom facility and that the problem persisted throughout the day of the audit. In turn, the auditor reviewed documents in your food safety manual and discovered that your food safety program did not designate a specific individual to monitor and supervise the cleanliness of your restroom facilities. As a result, the auditor completed a corrective action report (CAR) and failed your audit due to “automatic unsatisfactory conditions in all restrooms due to overflowing trash receptacles and trash on the floors.” In turn, you prepare your response plan.
Your Proposed Corrective Action Response Plan: I acknowledge that our employees are following appropriate handwashing procedures, as evidenced by the overflowing trash bins and paper scattered across bathroom floors. However, all restroom facilities must be maintained in a sanitary condition as a requirement for preventing the spread of bacteria. During our audit, it was discovered that all of our restroom facilities presented unsanitary conditions due to “overflowing trash receptacles and trash on the floors.” This unsanitary condition stemmed from a lack of established restroom maintenance procedures for our operation and resulted in the automatic failure of our audit on July 1, 2018.
To address the automatic unsatisfactory condition related to our restroom facilities, I propose the following short-term corrective actions: On July 1, 2018, I will assign eight individuals to immediately empty all trash bins, clean the floors and sanitize all of the restrooms. Further, I will designate our food safety officer (Jane Doe) to make sure the assigned staff have carried out all steps necessary to correct the immediate problem and that the task completion is recorded in our food safety manual. Ms. Doe will supervise ongoing efforts to maintain the cleanliness of the restrooms until we have a permanent restroom facilities manager in place. Because the nonconformity posed an immediate food safety threat to our operation, the short-term action steps proposed in this corrective action response plan were performed on the day of our initial audit, July 1, 2018.
To address the automatic unsatisfactory condition related to our restroom facilities, I propose the following long-term corrective actions: On July 2, 2018, I will create a permanent staff position to manage the cleanliness of all restroom facilities. I expect that the restroom facilities manager will be placed in position and fully trained to perform duties by July 9, 2018. Between July 2, 2018, and July 8, 2019, Ms. Doe will develop the necessary SOPs, policies, training procedures and maintenance tracking logs to ensure that all restrooms are cleaned regularly throughout each day and that the facilities remain adequately stocked with soap, toilet paper and paper towels at all times. Our restroom facilities manager will report directly to our food safety officer, Jane Doe.
If the auditor believes your corrective actions will reasonably address the nonconformities that resulted in audit failure, he or she likely will sign and finalize the CAR to indicate acceptance of your proposed actions and timetable for implementation. If proposed actions and the timetable do not constitute short- and long-term solutions to the problem, the auditor will request that you review, revise and resubmit a corrective action response plan for approval. Any corrective actions that are accepted will not be approved officially until the auditor returns for a follow-up onsite visit and has the opportunity to verify that your implemented actions provide evidence of long-term effectiveness.
On the follow-up visit, the auditor will check to see if changes stemming from the corrective actions have been written into your food safety plan. The CAR will become a permanent record of your audit history, and the problem areas likely will be revisited each time an auditor makes a visit to your operation, including audits that you schedule in the future.
Follow-up Audit
You can expect a follow-up audit if your operation’s initial audit or unannounced surveillance review(s) failed to meet GAP&GHP program requirements. You will need to address all nonconformities noted in your corrective action report (CAR) before scheduling a follow-up audit. The follow-up review follows the same process as the initial audit. Upon arrival, the auditor will make his or her presence known and immediately meet with your food safety officer and you. During this opening meeting, the auditor will clarify the purpose of visit and explain the scope(s) to be reviewed. The auditor will follow the GAP&GHP audit checklist and keep notes while reviewing your documents and records, interviewing employees, examining products and observing the procedures of your operation.
If your operation failed the initial audit review due to an “automatic unsatisfactory condition,” your follow-up audit will include a review of all scopes listed in the initial audit request. (See Scenario 1 below for a hypothetical example) If your operation failed to meet GAP&GHP program requirements because of low scores for some (but not all) of your reviewed audit scopes, your follow-up audit only will include a review of the scopes with the low scores. (See Scenario 2 below for a hypothetical example) Once the observation of your operation is complete, the auditor will work privately to review audit notes and findings, and tally your scores for the repeated and completed scope(s). The auditor will complete a corrective action report (CAR) if any nonconformities exist. You will have the opportunity to discuss the auditor’s findings and ask questions during the closing meeting. The auditor will provide a copy of the of the audit report during the closing meeting.
Follow-up Audit Scenario 1: Your initial audit consists of three GAP&GHP scopes: General Questions, Part 1 and Part 2. The auditor discovers that your field sanitation units are located in close proximity and uphill of your strawberry field, creating a possibility of spilled sewage running downhill into the crop production area. You passed the audit scopes for General Questions and Farm Review (Part 1) but failed the entire audit because of the automatic unsatisfactory condition for Part 2 (Field Harvest and Field Packing Activities, Questions 2-4). During your follow-up visit, your audit will consist of all three scopes: General Questions, Part 1 and Part 2.
Follow-up Audit Scenario 2: Your initial audit consists of three GAP&GHP scopes: General Questions, Part 1 and Part 2. To pass each scope, you need to score 80 percent of your total adjusted points. (See the audit scoring section on p. 29.) You scored 94 percent (160 out of 170 total adjusted points) for the General Questions scope; 78 percent (140 out of 180 total adjusted points) for the Farm Review (Part 1) scope and 92 percent (165 out of 180 total adjusted points) for the Field Harvest and Field Packing Activities (Part 2) scope. You have successfully passed the General Questions and Part 2 scopes. During your follow-up visit, your audit will consist of the Farm Review (Part 1).
Unannounced Verification Visits
Unannounced visits are an important component of the USDA GAP&GHP audit verification program. These visits coincide with an initial or follow-up audit that has been performed and successfully passed within a given season. The purpose of an unannounced review is for the auditor to verify consistency in your operation’s conformance with requirements of the USDA GAP&GHP program and with those of your food safety plan. As the name implies, you will not know specifically the date that the auditor will show up to perform surveillance. The number of minimum required unannounced verification visits to your location during a season is dependent on the audit scope(s) performed, the number of commodities grown in a production area during a given season and the number of days your operation has been in business.
Conditions for Part 1 Audit
Following a GAP&GHP audit for Part 1, you are not required to have unannounced verification visits if you grow only one commodity in a production area during the season (e.g., only potatoes or only onions, etc.). However, unannounced verification visits are applicable to your operation if you grow multiple commodities in the same crop production area in the same growing season. (See Table 6.)
|
Table 6. Number of Commodities Grown |
|
|
Number of Commodities |
Number of Required Visits |
|
1 |
0 |
|
> 1 |
1+ |
|
Note: Minimum* Number of Required Unannounced Visits, Based on Number of Commodities Grown in Same Crop Production Area During Season, Following a GAP&GHP Audit (Part 1). |
|
Conditions for Parts 2 through 6 Audits
Following GAP&GHPaudits for Parts 2 through 6, you are not required to have unannounced verification visits during the season if you have been in operation less than 31 days. However, if you have been in operation from 31 to 90 days, you can anticipate a minimum of one unannounced verification visit from the auditor during the season. If you have been in operation more than 90 days, you can expect to have at least two unannounced verification visits during the season. (See Table 7.)
|
Table 7. Days in Operation |
|
|
In Operation |
Number of Required Visits |
|
< 31 days |
0 |
|
31- 90 days |
1+ |
|
> 90 days |
2+ |
|
Note: Minimum* Number of Required Unannounced Visits, Based on Number of Days in Operation During Season, Following a GAP&GHP Audit (Parts 2, 3, 4, 6). |
|
*Note: In any situation, the USDA reserves the right for the auditor to conduct additional unannounced verification visits if there is suspicion that your operation is noncompliant with the requirements of the GAP&GHP program and/or your written food safety program.
Automatic Unsatisfactory Conditions
Any variety of situations may arise that will require the auditor to terminate an audit upon the discovery of unsatisfactory conditions. Although not meant as an exhaustive list, the USDA considers the following conditions as automatic grounds for audit failure:
- The farm does not have a written food safety plan in place or no one is designated to oversee the food safety program.
Your operation must have a documented food safety program in place. If you are missing this document, the auditor cannot verify that the implementation of your food safety program conforms to USDA GAP&GHP standards. Without a written plan, the auditor is obligated to terminate your audit. - No one is designated to oversee and implement the food safety plan.
You must designate an individual to oversee your food safety program, and this individual must be named in your food safety manual. The lack of a designated food safety officer demonstrates a lack of commitment to food safety. This unsatisfactory condition will be grounds for the auditor to fail your audit. - An immediate food safety risk is present when produce is grown, processed, packed or held under conditions that promote or cause the produce to become contaminated.
Your auditor will look for immediate food safety risks while reviewing your operation’s processes. If the auditor believes your processes have created product contamination or conditions that are likely to cause product contamination, your operation will not meet the minimum GAP&GHP program requirements, and the audit will be terminated. Examples of this would be the use of nonpotable water in the product washing process, a leaky sewer pipe in the production area or livestock found in the irrigation water. - There is presence or evidence of rodents, or an excessive amount of insects or other pests in the production area during packing, processing or storage.
The auditor immediately will stop the audit if the production or storage areas show evidence or infestations of rodents, birds or other mammal-type pests or show evidence of feces from various pests. An example of this condition would be the harvest of strawberries from a production area infested with rabbit droppings. - Observation of employee practices (personal or hygienic) that have jeopardized or may jeopardize the safety of produce.
Your employees must understand the threats to product safety when proper hygienic practices are not practiced on a constant basis. The following examples would cause the auditor to terminate your audit: An employee puts partially eaten product back into the product flow zone, spits on product or into product flow zones, disposes used toilet paper on the restroom floor or uses the toilet facility but does not wash hands before returning to work. - Falsification of records.
The USDA considers falsification of records an egregious offense that will result in an automatic failure of your audit. An example of this would be changing water test results or changing the dates of the actual test.
Audit Scoring
The auditor will refer to the checklist that corresponds to your audit scopes to evaluate your operation’s conformity to the GAP&GHP audit requirements and those of your food safety program. While a perfect audit score is obtainable, some minimal exposure to risk is assumed when you choose not to implement certain measures pertaining to the audit checklist items. However, the USDA audit standards are not meant to eliminate all risks. Rather, audit participation serves to validate your food safety plan as a way to reduce risk on your farm while achieving certification.
The goal of your audit is to demonstrate to the auditor that your operation has successfully implemented a food safety program that meets GAP&GHP audit requirements so that you may receive certification. You and your food safety officer should understand how the GAP&GHP audit checklist items are organized, how questions are structured and how the auditor will categorize the responses for tallying your audit scores.
Because the USDA GAP&GHP checklist is generic and can be used for any commodity and operation, there may be instances when a particular question does not pertain to your operation. In such a situation, the auditor will categorize the item as not applicable (N/A), provide a brief explanation in the comments section justifying the N/A designation and exclude the item from your audit score calculation. Additionally, the checklist contains some questions where designating N/A is not an option, and the auditor must designate either a “Yes” or “No” response. When any form of nonconformance is identified for any of the items, the auditor will include an explanation in your audit report to clarify the determination.
The auditor follows a sequential process to score the items. First, the auditor will ask to see specific policies (P) and/or records (R) when required by checklist items. Second, the auditor will rate the materials for the relevant checklist items using three categorical options: Yes (the policies and records conform to GAP&GHP standards), No (the materials do not conform to GAP&GHP standards) or N/A (the item cannot be answered).
Third, the auditor will convert some of the categorical ratings to a point value. The scored items will range in value from five to 15 points. You will receive all available points for each checklist item that the auditor marked “Yes” and zero points for each item marked “No” or “N/A.” With the exception of a food defense (Part 7) audit, there are never partial points awarded for partially completed or minimally acceptable procedures pertaining to any checklist items. Fourth, the auditor will tally your points within each scope reviewed. A passing score within a scope is defined as 80 percent of the possible points (or adjusted points if necessary) for a scope, with no automatic unsatisfactory conditions discovered.
Fifth, the auditor will identify whether or not you have passed all scopes reviewed, which is a necessary requirement for passing the entire audit. For example, if your entire audit comprised General Questions and Part 1, you will need to earn 80 percent of your adjusted total points for the General Questions and 80 percent of your adjusted total points for Part 1 to pass the entire audit. (See the hypothetical examples, Tables 8-13 for an understanding of how adjusted and lost points impact the outcome of audit scopes.)
Table 8. Hypothetical GAP Audit - Adjusted Points in the General Questions Scope.
|
Item |
Auditor Observations and Comments |
Points |
Code |
Type |
|
Implementation of a Food Safety Program |
||||
|
P-1 |
Has implemented GAPs that are documented in the food safety manual |
Yes |
D |
|
|
P-2 |
Jane Smith is named in the food safety manual as the designated food safety officer. She has completed trainings and is qualified for the job. |
Yes |
D |
|
|
Traceability |
||||
|
G-1 |
Has a documented traceability program in place. Records are maintained to accurately trace products. |
15 |
Yes |
D |
|
G-2 |
Has not yet completed a mock recall, but this is the farm’s first time applying for certification and a mock recall is not yet required. |
10 |
N/A |
R |
|
Worker Health and Hygiene |
||||
|
G-3 |
Maintains records for all drinking water and handwashing water test results. |
10 |
Yes |
R |
|
G-4 |
Has a policy on worker/visitor health and hygiene training. |
10 |
Yes |
P |
|
G-5 |
Training log shows that all staff have been trained on proper sanitation and hygiene practices. |
15 |
Yes |
D |
|
G-6 |
Employees and visitors are following good hygiene/sanitation practices |
15 |
Yes |
|
|
G-7 |
Employees who handle or package produce are washing their hands before beginning or returning to work. |
15 |
Yes |
|
|
G-8 |
Signs are posted to enforce handwashing practices for all staff before beginning or returning to work. |
10 |
Yes |
|
|
G-9 |
The house restroom is clean; portable restrooms in field sanitation facilities are clean and properly supplied with supplies and potable water. |
15 |
Yes |
|
|
G-10 |
Cleaning and service logs show that portable restrooms are adequately cleaned and serviced. |
10 |
Yes |
R |
|
G-11 |
Has a policy on smoking and eating areas. |
10 |
Yes |
P |
|
G-12 |
Has a policy for addressing workers with foodborne illness. |
15 |
Yes |
P |
|
G-13 |
Has a policy on how to deal with product/food surfaces that come in contact with blood. |
15 |
Yes |
P |
|
G-14 |
Has a policy on what workers do when they get hurt. |
5 |
Yes |
P |
|
G-15 |
Keeps copies of licenses for all personnel who apply regulated preharvest and/or postharvest materials. Logs are kept for all personnel trained on proper use of applying nonregulated materials. |
10 |
Yes |
R |
Codes: P (SOP); R (records); D (records + SOP); Yes (compliant); No (not compliant); N/A (not applicable).
Table 9. Pass/Fail Calculations for the Hypothetical GAP Audit - General Questions Scope
|
Calculations |
Total Points |
||
|
Total Points Available |
180 |
180 |
|
|
Subtract Points for N/A Questions* |
G-2 (-10 points) |
- 10 |
-10 |
|
Adjusted Total Points |
180 – 10 = |
170 |
|
|
Required Passing Score (80%) |
170 x 0.80 = |
136 |
|
|
Farm’s Calculated Points |
Based on “Yes” responses |
160 |
160 |
|
Pass/Fail ‘General Questions’ |
160 > 136 |
Pass |
* G-2: 10 points adjusted; No mock recall required since this is the farm’s first time applying for certification.
Table 10. Hypothetical GAP Audit - Points Lost in the Farm Review Scope.
|
Item |
Auditor Observations and Comments |
Points |
Code |
Type |
|
Water Usage |
||||
|
1-1 |
Uses pond as irrigation water source. |
|||
|
1-2 |
Crops are irrigated using sprinkler method. |
|||
|
1-3 |
Has water test results for water used for irrigation purposes. |
15 |
Yes |
D |
|
1-4 |
Has water test results for water used for chemical application. |
15 |
Yes |
D |
|
1-5 |
Has adequate steps in place to protect irrigation water from contamination. |
15 |
Yes |
|
|
Sewage Treatment |
||||
|
1-6 |
Farm sewage treatment/septic system functions properly with no runoff. |
15 |
Yes |
|
|
1-7 |
Farm is not adjacent to any municipal/commercial treatment facility or waste material landfill. |
10 |
Yes |
|
|
Animals/Wildlife/Livestock |
||||
|
1-8 |
Found poultry 20 feet away from crop production areas. |
15 |
No |
|
|
1-9 |
There are no manure lagoons on or near the property. |
10 |
N/A |
|
|
1-10 |
Manure is not stored near or adjacent to crop production area. |
10 |
Yes |
|
|
1-11 |
The pond is fenced to keep livestock out. |
10 |
Yes |
|
|
1-12 |
The fields have monitoring programs to track animal intrusion. |
5 |
Yes |
R |
|
1-13 |
Has fenced all of the crop production areas to minimize intrusions from domesticated and wild animals. |
5 |
Yes |
R |
|
Manure and Municipal Biosolids |
||||
|
Option B: Composted Manure |
||||
|
1-18 |
Composted manure and treated biosolids are used for soil amendments. |
10 |
Yes |
R |
|
1-19 |
Composted manure and treated biosolids are adequately treated. |
10 |
Yes |
D |
|
1-20 |
Composted manure and treated biosolids are properly stored to minimize recontamination. |
10 |
Yes |
|
|
1-21 |
Purchases composted manure from a supplier and has reports/test results. |
5 |
Yes |
R |
|
Soils |
||||
|
1-23 |
Land has been used for crop production for 20 years. |
5 |
Yes |
R |
|
1-24 |
Land use is commensurate with soil test results. |
10 |
Yes |
R |
|
1-25 |
Fields are not susceptible to flooding. |
5 |
Yes |
R |
|
Traceability |
||||
|
1-26 |
Production areas are coded for traceability in the event of a recall. |
10 |
Yes |
R |
Codes: P (SOP); R (records); D (records + SOP); Yes (compliant); No (not compliant); N/A (not applicable)
Table 11. Pass/Fail Calculations for the Hypothetical GAP Audit - Farm Review
|
Calculations |
Total Points |
||
|
Total Points Available |
190 |
190 |
|
|
Subtract Points for N/A Questions* |
1-9 (-10 points) |
- 10 |
-10 |
|
Adjusted Total Points |
190 – 10 = |
180 |
|
|
Required Passing Score (80%) |
180 x 0.80 = |
144 |
|
|
Farm’s Calculated Points** |
Based on “Yes” responses |
180 -15 = |
165 |
|
Pass/Fail ‘General Questions’ |
165 > 144 |
Pass |
* 1-9: 10 points adjusted because no manure lagoons located adjacent to the property.
** 1-8: 15 points lost because poultry was found 20 feet from crop production area.
Table 12. Hypothetical GAP Audit - Adjusted Points in Field Harvesting and Packing Activities Scope.
|
Item |
Auditor Observations and Comments |
Points |
Code |
Type |
|
Field Sanitation and Hygiene |
||||
|
2-1 |
Has a written preharvest assessment with details of the evaluation. |
15 |
Yes |
D |
|
2-2 |
The number, condition and placement of field sanitation units for this operation comply with state regulations. |
10 |
Yes |
|
|
2-3 |
Toilet facilities are readily available for all workers. |
10 |
Yes |
|
|
2-4 |
Field sanitation units are properly located and are accessible for servicing. |
10 |
Yes |
|
|
2-5 |
Has a written SOP for spills/leaks of field sanitation units and toilet facilities. |
10 |
Yes |
P |
|
Field Harvesting and Transportation |
||||
|
2-6 |
Has a policy and follows checklists to keep all harvesting containers and bulk hauling vehicles cleaned/sanitized to minimize contamination. |
10 |
Yes |
D |
|
2-7 |
Follows a checklist for regular cleaning and disinfecting of all harvesting equipment and implements (e.g., knives, pruners, etc.). |
10 |
Yes |
D |
|
2-8 |
Properly repairs or disposes all damaged harvesting containers. |
5 |
Yes |
|
|
2-9 |
All harvesting equipment and machinery is in good repair. |
10 |
Yes |
|
|
2-10 |
Operation does not use mechanical harvesting. |
10 |
N/A |
|
|
2-11 |
Has an SOP on glass/brittle plastic in the growing areas during harvest. |
5 |
Yes |
P |
|
2-12 |
Operation has an SOP in place to deal with product contamination from chemicals, petroleum, pesticides and other contaminating factors. |
5 |
Yes |
P |
|
2-13 |
Operation does not use mechanical harvesting. |
5 |
N/A |
|
|
2-14 |
Operation has a written policy that prohibits use of harvest containers for nonharvest purposes. All employees are trained on this policy. |
5 |
Yes |
P |
|
2-15 |
Water is treated to meet microbiological criteria; the treatment is approved and effective for its intended use and is appropriately monitored through regular testing. Keeps logs of test results. |
15 |
Yes |
R |
|
2-16 |
Employees are trained to wash and sanitize containers during harvest and remove excessive dirt/mud from produce and harvest containers. |
5 |
Yes |
|
|
2-17 |
All transportation equipment that comes in direct contact with product and that is used to move product from the field is clean and in good repair. |
10 |
Yes |
|
|
2-18 |
Has an SOP that all harvested product is covered during transportation. |
5 |
Yes |
P |
|
2-19 |
Operation does no field packing. |
10 |
N/A |
D |
|
2-20 |
Operation does no field packing. |
10 |
N/A |
|
|
2-21 |
Uses lot codes to identify product coming out of the field. |
10 |
Yes |
D |
Codes: P (SOP); R (records); D (records + SOP); Yes (compliant); No (not compliant); N/A (not applicable).
Table 13. Pass/Fail Calculations for the Hypothetical GAP Audit - Field Harvesting / Packing Activities.
|
Farm A |
Calculations |
Total Points |
|
|
Total Points Available |
185 |
185 |
|
|
Subtract Points for N/A Questions* |
2-10, 13, 19, 20 (-35 points) |
- 35 |
-35 |
|
Adjusted Total Points |
185 – 35 = |
150 |
|
|
Required Passing Score (80%) |
150 x 0.80 = |
120 |
|
|
Farm’s Calculated Points** |
Based on “Yes” responses |
150 |
150 |
|
Pass/Fail ‘General Questions’ |
150 > 120 |
Pass |
* 2-10, 2-13: 15 points adjusted because operation does no mechanical harvesting.
* 2-19, 2-20: 20 points adjusted because operation does no field packing.
Audit Checklists
This section covers the checklist items that auditors review when conducting the USDA GAP portion of the GAP and GHP audit. More specifically, our discussion focuses on eight preliminary questions and checklist items pertaining to the GAP program scopes: General Questions, Farm Review (Part 1), and Field Harvesting and Field Packing Activities (Part 2). We do not cover the checklist items for House Packing Facility (Part 3), Storage and Transportation (Part 4), Wholesale Distribution Center/Terminal Warehouse (Part 6), or the optional scope: Preventative Food Defense Procedures (Part 7). However, you may access the entire GAP&GHP audit checklist at https://www.ams.usda.gov/sites/default/files/media/GAPGHP_Checklist_no_spell_Checklist_Enabled%5B1%5D.pdf. In Section 5, you will find explanations of the checklist items and tips of what auditors may look for when evaluating your operation’s conformity to the USDA GAP&GHP standards and your food safety program.
Preliminary Questions
(Items 1 through 8)
At the beginning of the USDA GAP&GHP audit checklist, the auditor will review eight questions before digging into the General Questions scope.
- Did (you) participate in any GAP&GHP training? (Yes, No)
Audit Tip: Participation in GAP&GHP training can be demonstrated in a number of ways such as attending Cooperative Extension workshops, formalized classes, GAP-based seminars or other professional trainings that focus on water, manure/biosolids, worker health and hygiene, sanitary field facilities, field sanitation, packing facility sanitation or transportation issues. - Is there a map that accurately represents (your) farm operation? (Yes, No, N/A)
Audit Tip: Your manual should include some type of map that reflects your growing areas. You may need to have multiple maps if your operation is large. Your map(s) should define specific areas for all products that you grow. This information is pertinent to audit scopes Part 1 and Part 2. This information also is relevant to the review of your product traceback capabilities. - Legal Description of your Location: ___________
Audit Tip: Your legal description can be a county-issued parcel number (APN) or a township and range for the operation. Include information about wells and locations of former landfills that may be near or on the property, as the auditor will review this during the audit of your operation. - Are all (of your) crop production areas located on the audit site? (Yes, No, N/A)
Audit Tip: Full disclosure of all crop production areas is required for the auditor to properly evaluate Parts 1 and 2 of the GAP&GHP audit. The auditor needs to visit and observe the primary and secondary crop production locations to confirm the extent that each site is adhering to the GAP practices in a uniform manner. Each production location must meet the minimum GAP&GHP audit requirements. Failure at one site will disqualify the entire operation from passing the audit. - Total acres farmed ___________________
Audit Tip: This information helps the auditor determine what will need to be reviewed during a Farm Review (Part 1). Report all acres associated with your farming operation, including those that you own, lease, rent, contract or consign. The auditor will need to plan accordingly to visit all production areas covered by the audit. - Does (your) operation have more than one packing facility? (Yes, No, N/A)
Audit Tip: Prior to the start of your audit (Part 3), designate whether you are grouping multiple packing facilities into a single audit or prefer to conduct separate audits for each facility. When using a blanket audit for all facilities, understand that failure in one facility to meet audit standards will result in failure across all of your facility locations. When audited separately, a problem in one of your facilities will not affect the final audit of the other facilities. - Is there a floor plan of (your) packing house facility indicating flow of product, storage areas, cull areas, employee breakrooms, restrooms and offices? (Yes, No, N/A)
Audit Tip: When your audit scope covers Parts 3 or 4, your floor plan helps the auditor quickly locate potential problem areas within the packing house facility that need to be observed and evaluated. Your floor plan should indicate the flow of the product storage area, where the product enters and leaves the facility, break areas, offices, etc. Identify where pest control devices and water lines are located, and include maps of your farm/field(s) that show surrounding irrigation sources, hydrants, etc. - Is any (of your) product commingled prior to packing? (Yes, No)
Audit Tip: Your operation may commingle produce with product coming from different growers. This commonly occurs at the storage level prior to packing, during processes of pregrading and presizing, or at the time of packing. Track any commingling that occurs on your farm because the process directly impacts your ability to trace products that may need to be recalled. The auditor will refer to this information when assessing questions pertaining to Traceability.
General Questions
Within the General Questions scope, 17 questions cover three main topics: (a) Implementation of a food safety program (items P-1, P-2), (b) traceability (G-1, G-2) and (c) worker health and hygiene (G-3 through G-15). The General Questions scope is mandatory for all GAP&GHP Parts 1 through 4, and 6. As we walk through the three topic areas, keep in mind that you must earn 80 percent of your adjusted total point value for the General Questions section or the auditor will terminate the audit.
Implementing a Food Safety Program (Items P-1 and P-2)
P-1. (Your) documented food safety program that incorporates GAP and/or GHP has been implemented. (Yes, D)
Audit Tip: This question requires the auditor to review your food safety manual for documented evidence that you have (at minimum) a GAP&GHP program established for the scope(s) being audited. Your manual must provide sufficient evidence that you have an established food safety program in place. The auditor will review policies and records for proof that your food safety program is being implemented as written in the manual. At minimum, your standard operation procedures (SOPs), records and supporting documentation should cover the scope(s) being audited. As an example, if you have performed internal or self-audits of your food safety program, be sure your food safety manual includes information and references to these activities. Overall, if the auditor cannot mark this question with a “Yes” response, you automatically will fail the audit.
P-2. (Your) operation has designated someone to implement and oversee your food safety program. Name: __________________ (Yes, D)
Audit Tip: This question requires the auditor to review your food safety manual for documented evidence that you have a designated and qualified individual in place to oversee the implementation of your food safety program. As an example, your food safety officer can be named in an organizational chart of your operation. The auditor will interview your food safety officer to determine his/her knowledge of your program as it pertains to the scope(s) being audited. When interacting with the auditor, your food safety officer should be able to demonstrate procedures, provide records and answer questions that the auditor may have about the food safety program. If the auditor cannot mark this question with a “Yes” response, you will automatically fail the audit.
Traceability (Items G-1 and G-2)
Your food safety program must include a documented traceability program. Having traceability procedures in place can help speed the process of pinpointing a source of food contamination in the event of an illness outbreak. The faster the source is identified, the faster associated problems can be addressed, which, in turn, helps to restore public confidence in the industry as a whole. Within the General Questions scope, the auditor will evaluate your product tracking capabilities using two checklist items.
G-1: A documented traceability program has been established. (Yes, No, D)
Audit Tip: This question requires the auditor to review your food safety manual for documented evidence that you have a program in place that allows a traceback investigation to follow your product(s) forward and backward at least to the next level in the marketing chain. Simply stated, a traceability program reflects the ability to know where products are received from and where they are sent. You will not meet traceability requirements if your methods for tracing and identifying product(s) focus on one direction of the marketing chain (e.g., forward) and ignore the other (e.g., backward).
The auditor must be able to find a written procedure in your food safety plan that describes how you are able to track individual containers of your products within the marketing chain. For traceability purposes, maintain as many detailed records as possible to include harvest date(s), specific fields (or orchards), the number of packages within a lot, packing and shipping dates, harvesting crews, etc. Such documents may be in paper or electronic form, and must be accessible during an audit, as well as during an actual traceback investigation.
The farm address on a box may be sufficient to trace contaminated product back to your farm or packing house. Each wholesale box must have a harvest/packing date stamp or code with the date physically incorporated on each box. Simply sticking a label on a wrapped pallet is inadequate. If packing occurs in more than one shed or packing is under another producer’s label, the auditor will look for additional information that enables quick and accurate tracing of product(s) back to the packing house. When your product is commingled, the auditor will assess the extent that your product can be traced to a reasonably sized group of growers (e.g., five to eight growers for tree fruits, three to five for vegetables) and/or traced to a harvest period that is less than eight days. Your product container markings must be reliably applied and easily read or deciphered at any point up and down the marketing chain.
Your on-farm traceability program must:
- Track the product to the grower and production area from which it came
- Include your production area(s), whether you have one or more orchards/fields.
- Include production records that show the grower, production area and year
- Document harvest dates
- Document all growers, production areas and dates for product that is comingled during or after harvest
- Include crop production records, farm maps, transportation bills, weight tickets and storage records
- Document where a product has been sent once it leaves your facility.
At the packing stage and forward in the marketing chain, your traceability program must:
- Include sufficient identification on products/packages/containers to allow tracing to a packing house
- Mark product or individual containers in a unique manner so that product can be traced back to its originating facility if there are multiple packing facilities
- Identify some form of “pack date” on all packed containers
- Document pack dates that correspond to your farm’s traceback information.
For additional guidance on developing a traceability program, contact trade/commodity associations, state horticultural associations or county Extension agents.
G-2: The operation has performed a mock recall that was proven to be effective. (Yes, No, R)*
* Note: if your audit is specific to the scopes of the farm review or harvest, a mock recall is not required during your first year’s participation in the USDA GAP and GHP program.
Audit Tip: This question requires the auditor to verify that your mock recall will effectively work in the case of a real recall event. The auditor will review your records to determine how well you are able to quickly identify, trace and recall implicated product(s) when the need arises. To prepare for the worst, perform a recall simulation each year and ensure that your system works efficiently. When conducting the mock recall, document the customers that were contacted, the amount of product remaining from the original shipment and the amount of product that could not effectively be recalled.
A recall provides the means to remove product from market and return the product its origin.
A mock recall is a practice exercise used to identify where product has been shipped and the extent that it can be returned or removed from the marketing chain.
A recall program is the ability to pull products from the marketplace once they have left the operation’s control.
The auditor will look for your procedures to clearly explain how you want your recalled products to be handled. Do you want the product returned to you? Will you allow buyers to dispose of it and submit proof that your products have been dumped? You will need to communicate your policy to buyers so they know how to effectively manage the inventory of products they have purchased from you.
Worker Health and Hygiene (Items G-3 through G-15)
A primary food safety risk associated with fruits and vegetables is the potential introduction of pathogens through poor worker health and hygienic practices. Good hygiene protects workers from illness and reduces the potential contamination of fresh produce that eventually ends up in the hands and mouths of consumers. Worker health and hygiene is addressed through 13 questions in the General Questions section (G-3 through G-15), although additional questions pertaining to worker hygiene practices can be found in four other audit scopes: Parts 2, 3, 4 and 6.
G-3: Potable water is available to all workers. (Yes, No, R)
Audit Tip: This question requires the auditor to review your water test results and records to determine whether or not your potable water is safe for your workers to drink or suitable for washing hands or other body parts. The test results for drinking water should show levels of microbial content that are within the EPA, state or local guidelines. To reduce contamination, you may find it necessary to add chlorine or other acceptable agents to the potable water meant for washing hands or other body parts. The auditor will look at these test results and verify that treated potable water for washing is not used for drinking purposes.
G-4: All employees and all visitors to the location are required to follow proper sanitation and hygiene practices. (Yes, No, P)
Audit Tip: This question requires the auditor to review your policies and records to determine whether or not you have an established policy on sanitation and hygiene practices, what it covers, how you enforce it, and the extent that it is being followed by employees and visitors. The auditor will look for policy indicators such as signs posted to instruct visitors and employees to follow established sanitation and hygiene practices (including handwashing), sign-in or check-in steps that people must follow as they enter your premises, the use of a specific door or an office through which people must enter to reach certain areas and attire/dress codes that must be followed while in specific areas of your operation. Hold your auditor to the same sanitation and hygiene standards as required for visitors.
Minimal statements such as in employee handbooks, printed signs at various locations or directions during orientation will not provide the auditor sufficient evidence that your SOPs demonstrate a required process. The auditor will look for your policy to require regular and periodic reviews of employee/visitor habits, training sessions, required attire/dress codes, etc.
G-5: Training on proper sanitation and hygiene practice is provided to all staff. (Yes, No, D)
Audit Tip: This question requires the auditor to review your documentation, records and SOPs to determine the adequacy of your training program that is completed by staff. The auditor will review your policies and records to verify that (a) you have offered the required training, (b) you have documented when the training occurred, (c) you have performed any follow-ups beyond the training sessions, and (d) whether or not all employees involved with handling produce have completed the necessary training(s).
You will need to ensure that all personnel comprehend the food safety impact of poor personal cleanliness or unsanitary practices. It is your responsibility to develop a sanitation training program for your employees and make sure they are educated about GAPs and GHPs. Simple statements at the beginning of a season that direct employees to follow safe food handling practices will not satisfy the totality of the training requirement, nor will the inclusion of a written policy in an employee orientation or policy manual. As examples of in-depth training evidence, activities would include formal presentations, one-on-one instructions and demonstrations (e.g., handwashing). Provide periodic refresher or follow-up training sessions with your staff throughout the year. All of your training activities should be documented and suitable to the number of attendees and the job experience of employees. The auditor will review your documentation and verify the positive attempts to provide training to all staff.
G-6: Employees and visitors are following good hygiene/sanitation practices. (Yes, No)
Audit Tip: This question relates to G-4 and G-5, and requires the auditor to observe various hygiene and sanitation practices of employees and visitors throughout the audit day. Across all observations, the individuals must be following acceptable or company-required practices or the auditor will mark this question as “No.”
G-7: Employees who handle or package produce are washing their hands before beginning or returning to work. (Yes, N/A, D)
Audit Tip: This question applies to employees working directly with produce and does not apply to employees involved with pruning or other similar fieldwork. The auditor will review your policies and records to verify that your operation enforces proper handwashing practices of employees involved with handling or packaging produce. Information printed in employee handbooks or a simple policy statement will not provide the auditor sufficient evidence to demonstrate a required process unless there is documented evidence of periodic reviews and follow-ups of employees’ hygiene practices. Additionally, the auditor will observe those involved with handling and packaging food products and verify whether or not they are following proper handwashing practices before beginning or returning to work.
Thorough handwashing before beginning work with produce and after using the toilet is critical to reducing the spread of bacteria and food contamination. Many diseases transmissible through food originate in the intestinal tract of employees and shed through their feces. Contaminated hands, including poorly cleaned fingernails and cuticles, also can aid disease transmission.
Recommended Handwashing Procedure:
- Wet hands with clean potable water, apply soap and work up a good lather.
- Rub hands together for at least 20 seconds.
- Clean under nails and between fingers. Rub fingertips of each hand in suds on palm of opposite hand.
- Rinse under clean running water.
- Dry hands with a single-use towel.
The auditor will not mark this question as “Yes” if employees use sanitizer alone and do not wash hands with soap or another agent. If the auditor cannot answer this question with a “Yes,” your audit will be reported as an automatic unsatisfactory condition.
G-8: Readily understandable signs are posted to instruct employees to wash their hands before beginning or returning to work. (Yes, No, N/A)
Audit Tip: This question requires the auditor to look for understandable signs to be posted near your handwashing stations. The auditor will question and observe employees to determine the extent that the practice is understood and followed.
Your signs should require that employees wash hands before going to and returning to work following an activity that is other than handling produce (e.g., regular breaks, meal breaks, etc.). If you have non-English speaking employees on staff, post signs in the native language of the predominant number of workers. Graphic signage that demonstrates and reminds workers to follow the handwashing requirement would be acceptable.
Your signs must be posted within close proximity of the handwashing stations for the auditor to score them as adequate policy reminders. The auditor will only score this question as N/A when toilet facilities are not required or present for your operation (e.g., when there are less than the minimum number of workers present or in-home toilets are used only by family members).
G-9: All toilet/restroom/field sanitation facilities are clean. They are properly supplied with single-use towels, toilet paper, and hand soap or anti-bacterial soap and potable water for handwashing. (Yes, No, N/A)
Audit Tip: This question requires the auditor to review all toilet/restroom facilities pertinent to your audit. The handwashing facilities must be located near toilet facilities that are frequented by food-handling employees so that these individuals have ample opportunity to wash before starting or returning to work. You must provide handwashing facilities with soap and towels, either inside or outside of the toilet facility.
The auditor will make observations throughout the day of the audit to draw factual conclusions of your toilet/restroom/field sanitation facilities. The auditor will consider the number of people using the facilities, time of day that his/her observations were made, your cleaning schedules and the overall appearance of facilities. While a few pieces of paper here and there would be acceptable, a pile of used towels spilling out of the trash receptacles and onto the floor would not be. (See example, Figure 7, p. 25.) Used toilet tissue found on the toilet room floor or in a toilet room container will be grounds for audit failure. If the auditor concludes that any one toilet facility within your operation does not meet the minimum hygiene and sanitation requirements, your audit will be reported as an automatic unsatisfactory condition. The auditor may only score this question as N/A when toilet facilities are not required or present for your operation.
G-10: All toilet/restroom/field sanitation facilities are serviced and cleaned on a scheduled basis. (Yes, No, N/A, R)
Audit Tip: This question requires the auditor to examine and crosscheck your records with his/her observations made at the toilet/restroom/field sanitation unit facilities. Your food safety plan must specify a cleaning schedule for all toilet/restroom/field sanitation unit facilities of your operation. If you contract with an outside vendor, the auditor will review service contracts and records for proof that the toilet/restroom/field sanitation units are adequately supplied and regularly cleaned. The auditor may only score this question as N/A when toilet facilities are not required or present for your operation.
G-11: Smoking and eating are confined to designated areas separate from where product is handled. (Yes, No, N/A, P)
Audit Tip: This question requires the auditor to review your policies regarding smoking and eating activities within your operation in relation to where food packing and storage are being completed. For the field-handling areas, smoking and eating activities should be confined to the edges of the field that are out of the harvesting zone or in drive areas between fields. In the packing or storage facilities, the designated areas for such activities should be sufficiently distanced from the flow-zone areas and clearly marked as designated areas (e.g., with painted lines or partitions). Employees may drink bottled water in the work area if the water is stored in closed plastic containers away from the product flow zone when the bottles are not being used. If the auditor discovers any other signs of eating, smoking or the presence of food or tobacco items in a food-handling or storage area, the auditor will mark this question as “No.”
G-12: Workers with diarrheal disease or symptoms of other infectious disease are prohibited from handling fresh produce. (Yes, No, P)
Audit Tip: This question requires the auditor to review your policies to determine whether or not your operation prohibits ill employees from handling fresh produce or food packages, or working around product flow zones. Instruct employees to report any active cases of illness to the shift managers/supervisors prior to starting work. Your managers/supervisors must be familiar with infectious diseases, be able to recognize when employees display symptoms and have the authority to take appropriate steps when illnesses are suspected. The auditor will pose questions of your managers/supervisors to determine their knowledge of infectious disease symptoms and their ability to identify symptoms in employee behaviors. Additionally, the auditor will look for indications of worker illness during the audit (e.g., prescriptions, over-the-counter antidiarrheal medications, frequent trips to the toilet facilities by individual employees, etc.).
G-13: There is a policy describing procedures that specify handling/disposition of produce or food contact surfaces that have come into contact with blood or other bodily fluids. (Yes, No, P)
Audit Tip: This question requires the auditor to review your SOPs and verify that you have written procedures for properly handling and disposing of any food products that have come in contact with blood or bodily fluids (whether from human or animals). Your policy also must describe what needs to be done to properly clean/sanitize food contact surface areas that have been contaminated. The auditor will review your food safety manual to determine the existence of policy, the adequacy of written procedures and whether or not you have any documented instances where the policy has been enforced.
G-14: Workers are instructed to seek prompt treatment with clean first-aid supplies for cuts, abrasions and other injuries. (Yes, No, P)
Audit Tip: Instruct your workers to report all conditions or injuries that could contaminate food products or containers. All injuries must be properly treated and covered before the injured individual returns to work around food or food contact surfaces. The auditor will verify this policy by reviewing your company documents and interviewing supervisory personnel or food handlers to see what they do in such instances.
G-15: Company personnel or contracted personnel who apply regulated preharvest and/or postharvest materials are licensed. Company personnel or contracted personnel applying nonregulated materials have been trained on its proper use. (Yes, No, N/A, R)
Audit Tip: If this question pertains to your audit and operation, the auditor will review your records to ensure that you use authorized and trained individuals to apply regulated and/or nonregulated preharvest and/or postharvest materials. All personnel involved in the material applications must have sound working knowledge of the use of preharvest materials (e.g., pesticides, growth regulators, fertilizers) and post-harvest materials (e.g., waxes, fumigants, fungicides, etc.). Additionally, these individuals must know the appropriate strength levels and what to do if levels and strengths are improperly mixed or if mixtures are spilled. If your applicators do not hold current federal or state licenses, the auditor will review training documents for proof that such personnel have received training on proper use of the materials.
Farm Review
All questions covered in the General Questions scope are applicable to your farm review scope. The Farm Review (Part 1) audit checklist includes up to 21 questions that cover water usage (Items 1-1 through 1-5), sewage and its treatment (1-6 and 1-7), the presence of animals and livestock (1-8 through 1-13), use of manure and biosolids (Option A: 1-14 through 1-17; Option B: 1-18 through 1-21; or Option C: 1-22), land use history (1-23 through 1-25) and traceability (1-26).
Water Usage (Items 1-1 through 1-5)
The quality of your water, how and when you use it and the characteristics of the crops that you grow have a direct impact on potential microbial contamination in the produce entering the food chain. Inadequate water quality can be a contamination source and a vehicle for spreading microbial contamination in production areas, harvesting and packing facilities, or transportation environments.
1-1: What is the source of irrigation water? (Pond, Stream, Well, Municipal, Other): __________________.
1-2: How are crops irrigated? (Flood, Drip, Sprinkler, Other): ___________________________.
Audit Tip: To answer questions 1-1 and 1-2, the auditor will list all of your water sources and specify which methods you use to irrigate your crops.
1-3: A water quality assessment has been performed to determine the quality of water used for irrigation purposes on the crop(s) being applied. (Yes, No, N/A, D)
1-4: A water quality assessment has been performed to determine the quality of water used for chemical application or fertigation method. (Yes, No, N/A, D)
Audit Tip: For checklist items 1-3 and 1-4, the auditor is required to review your irrigation and spray methods, determine whether you have (or have not) performed risk assessments of water quality and whether any water you have used was suitable for spray or irrigation. Questions 1-3 and 1-4 will be answered N/A if you do not use irrigation or spray applications on your crop.
You must know your water quality to determine potential risks for product contamination through irrigation or chemical spraying. Drip irrigation methods are less likely to promote contamination compared to flood irrigation methods. Sprinkler methods pose the most risk, especially if your farm’s water quality is unknown. Potable water is not required for chemical applications or irrigation that occurs prior to planting your crops or if crops are dormant. However, you must use microbial-safe water when applying chemical and irrigation applications just prior to crop harvest.
The auditor will look for policies and records as proof that you have performed water-testing risk assessments at the appropriate time(s) in the season and on a regularly scheduled basis. Municipal water should be tested each year by the local water authority. Well water should be tested at least once a year and treated if fecal coliforms are present. Surface water should be tested three times during the growing season in North Dakota: at first planting, at peak use, and at or near harvest.
1-5: If necessary, steps are taken to protect irrigation water from potential direct and nonpoint source contamination. (Yes, No, N/A)
Audit Tip: The auditor’s assessment of internal and external sources of contamination in questions 1-3 and 1-4 will determine your need to take steps to protect water. If this is your situation, the auditor will review documentation or observe your premises to see if you have taken sufficient steps to maintain a quality water source. If polluted runoff is a potential risk to your production areas, the auditor will look for the use of berms, swales, diversions, etc., and check for backflow prevention devices when required by code or evaluation. Question 1-5 only will be answered N/A when you have no further need to protect the water supply or do not use water to irrigate, fertigate or spray products.
Sewage Treatment (Items 1-6 and 1-7)
Depending on your location, you may be treating your farm sewage through a municipal system or a septic system. Municipal systems have regular testing and sewage treatment procedures in place, while septic systems near a water source pose greater contamination potential, especially when the water source is not sealed. Typically, farms rely on simple septic tank systems that consist of a holding tank for solids and leach lines for liquids.
1-6: The farm sewage treatment system/septic system function properly, and there is no evidence of leakage or runoff. (Yes, No, N/A)
Audit Tip: This question requires the auditor to verify that your septic tank system is sealed and that there are no leaks near your tank or within the leach field. If your system is functioning properly, the auditor will answer “Yes” to this question. Similarly, if you have a public sewage line with no evidence of leaking, the auditor will mark this question with a “Yes.” The auditor may mark this question as N/A should your operation not require sewage treatment, such as with a field location that has no standing buildings or sewage treatment connections on site.
1-7: There is no municipal/commercial sewage treatment facility or waste material landfill adjacent to the farm. (Yes, No, N/A)
Audit Tip: To mark this question “Yes,” the auditor must verify that your farm is not adjacent (within ¼ mile) to a municipal or commercial sewage treatment facility.
Animals/Wildlife/Livestock (Items 1-8 through 1-13)
The USDA GAP&GHP program requires operations to monitor for signs or the presence of domesticated and wild animal intrusions in crop production areas and to take steps to reduce animal entry into such areas. It will be difficult to control wild animal intrusions, especially when crop production areas are near wooded areas, open meadows or waterways. Although total exclusion is not possible for wild animals, make every effort to limit animal access to reduce potential sources of contamination for fruit and vegetable crops.
1-8: Crop production areas are not located near or adjacent to dairy, livestock or fowl production facilities unless adequate barriers exist. (Yes, No)
Audit Tip: This question requires the auditor to observe the distance between your crop production areas and the presence of potential contamination sources, for example, dairy or livestock production facilities, including feedlots. The auditor will mark this question “No” if your crop production area is less than one mile from an animal production area and no natural barriers exist.
1-9: Manure lagoons located near or adjacent to crop production areas are maintained to prevent leaking/overflowing or measures have been taken to stop runoff from contaminating the crop production areas. (Yes, No, N/A)
Audit Tip: To mark this question “Yes,” the auditor must verify that you have taken measures to prevent nearby manure lagoons from leaking or overflowing into your crop-growing areas. Such measures may include physical barriers such as ditches, mounds, grass/sod waterways, diversion berms, etc. When your farming operation is on higher elevation ground than the lagoon, the elevation will be a natural barrier. The auditor will mark this question as N/A when there are no manure lagoons near or adjacent to your production areas.
1-10: Manure stored near or adjacent to crop production area is contained to prevent contamination of crops. (Yes, No, N/A)
Audit Tip: Manure is considered a major source of potential contamination. To mark this question “Yes,” the auditor must verify that your manure storage areas are constructed to contain any potential leaching and runoff from entering your crop production areas.
1-11: Measures are taken to restrict access of livestock to the source or delivery system of crop irrigation water. (Yes, No, N/A)
Audit Tip: This question requires the auditor to determine whether or not you have taken measures to keep livestock out of the water supply sources (e.g., wellhead area or pond/stream) or delivery systems (canal/ditch) that are used to irrigate your crops. Keep livestock at least 200 feet from the water source when you have only a few head of livestock and access to the source is random. The auditor will mark this question as N/A when your farm has no livestock present or when dairy/livestock facilities are not adjacent to your farm.
1-12: Crop production areas are monitored for the presence or signs of wild or domestic animals entering the land. (Yes, No, N/A, R)
Audit Tip: This question requires the auditor to review your records and verify that you are monitoring your crop production areas for the entry of domesticated or wild animals. Examples of documented monitoring efforts could take the form of regularly completed notes, scouting lists or crop maintenance reports. Although the surveillance activities do not need to occur on a daily basis, the auditor will look for records to reflect a regular schedule that demonstrates your awareness of the animal populations in the production areas. This question can only be marked as N/A for fully enclosed greenhouses.
1-13: Measures are taken to reduce the opportunity for wild and/or domestic animals from entering the crop production areas. (Yes, No, N/A, R)
Audit Tip: This question requires the auditor to review your records and verify that you have taken steps to minimize animal entry into your crop production areas. The auditor will look for deterrence indicators such as the placement of noise cannons or scare balloons to keep away birds and migratory waterfowl, or fencing or other barriers that limit wildlife access. The auditor will review your documentation pertaining to the control of potential contamination from farm service animals (horses, oxen, mules) in the production areas and will evaluate your remediation steps taken when any contamination has occurred. The auditor will mark this question as “No” if he or she finds no evidence of any positive attempts to deter frequent visits of wild or domesticated animals into your crop production areas. This question will only be considered N/A if your monitoring records show that animal intrusion is very infrequent or nonexistent. The auditor will automatically mark Item 1-13 as N/A if item 1-12 has been marked as N/A.
Manure and Municipal Biosolids (Items 1-14 through 1-22)
Animal manure and biosolids are significant sources for potential contamination. Left untreated, any raw animal manure or raw biosolids that are used as fertilizers or to improve soil structure, or that enter surface or ground water through runoff, may contain pathogens that pose a significant public health risk. However, when properly treated, manure and biosolids can be effective and safe fertilizers for your operation.
Crops in or near the soil are most vulnerable to pathogens that may survive in the soil. Pathogens in manure may persist in the soil, and low-growing crops have a higher risk for contamination if splashed with soil during irrigation or heavy rainfalls. If you use manure or biosolids, your operation is required to perform risk assessments to determine if and when raw manure or biosolids are appropriate for use. If you use manure or biosolids, you are required to take steps to minimize microbial hazards.
The auditor will choose one of three options that reflects your operation’s use of soil amendments: (a) raw manure or a combination of raw and composted manure is used as a soil amendment (items 1-14 through 1-17), (b) only composted manure/treated municipal biosolids are used as a soil amendment (items 1-18 through 1-21) or (c) no manure or municipal biosolids of any kind are used as a soil amendment (item 1-22).
Option A: Raw Manure
1-14: When raw manure is applied, it is incorporated at least two weeks prior to planting. (Yes, No, R)
1-15: Raw manure is not used on commodities that are harvested within 120 days of planting – if the edible portion does come in contact with the soil and 90 days if the edible portion does not come in direct contact with the soil. (Yes, No, R)
To answer questions 1-14 and 1-15, the auditor is required to review manure application records and determine whether or not raw manure has been properly used according to the recommendations. Be sure your records reflect documentation of rates, dates and location of manure applications. If you need to apply manure or slurry to vegetable or fruit soil, do so at least two weeks prior to planting and observe the 90/120-day preharvest interval. When you are unable to adhere to the 90/120 waiting period, apply only properly composted manure.
1-16: If both raw and treated manure are used, the treated manure is properly treated, composted or exposed to reduce the expected levels of pathogens. (Yes, No, N/A, R)
This question requires the auditor to review records to verify that the manure you have used has been properly treated to kill pathogens. The auditor may answer this question as N/A if no composted/treated material is used by your operation. (Refer to checklist item 1-18 for composted manure.)
1-17: Manure is properly stored prior to use. (Yes, No, N/A)
This question requires the auditor to investigate and verify that your untreated manure is properly stored in a manner that prevents leaching or runoff into adjacent crop production areas. Additionally, the auditor will verify that your storage of raw manure will not contaminate any storage of treated manure.
Option B: Composted Manure
1-18: Only composted manure and/or treated biosolids are used as a soil amendment. (Yes, No, R)
This question requires the auditor to review records and observe your operation for evidence that only composted manure or biosolids are used within your operation. If the auditor finds the use of any raw manure, the questions for Option A (raw manure) will be answered instead of Option B (composted).
1-19: Composted manure and/or treated biosolids are properly treated, composted or exposed to environmental conditions that would lower the expected levels of pathogens. (Yes, No, D)
This question requires the auditor to review documentation to verify that your product has been sufficiently treated to reduce pathogen levels. For example, the auditor will look at your time charts for passive treatments, or at time and temperature charts, or process explanations and microbial testing reports for active treatment methods.
1-20: Composted manure and/or treated biosolids are properly stored and protected to minimize recontamination. (Yes, No, N/A)
When products used to fertilize crops are delivered for future application, properly store the products in a manner that reduces recontamination and the likelihood of contamination in production areas or adjacent fields. Examples of barriers that can be used to prevent runoff, leaching or wind spread may include concrete block or soil berms, pits and lagoons. Separate the stored materials from irrigation, spray dilution or processing water sources. Consider covering manure piles, such as under a roof, to protect stored manure from rainfall that can result in leachate. Alternatively, you may want to collect water that leaches through the stored manure to control the disposal of the leachate. If you use the manure leachate in production areas, maximize the time between application and harvest to minimize microbial hazards. Prior to material application, the auditor must be able to complete a site review when manure or biosolid materials are stored on site.
1-21: Analysis reports are available for composted manure/treated Biosolids. (Yes, No, R)
This question requires the auditor to review specification sheets pertaining to sourced manure to clarify the method of treatment used by the manure supplier.
Option C: No Manure/Biosolids Used
1-22: No animal manure or municipal biosolids are used. (Yes, No, P)
This question requires the auditor to verify your policy on manure use. The auditor will only select Option C if your operation does not use raw or treated manure or biosolids. If the auditor finds any evidence of raw manure use by your operation, Option A will be selected. If the auditor finds evidence that you use composted manure and not raw manure, Option B will be selected.
Soils (Items 1-23 through 1-25)
Your operation must maintain documentation of land use and land use history for all crop production areas and adjacent properties. Testing must be performed in the crop production areas to verify that you have addressed all potential risks identified as likely to cause chemical, physical or microbiological contamination of produce. Mitigation strategies need to be put in place to minimize contamination coming from adjacent lands, whether the property is owned by the operation or not.
Document any flooding that is caused by the overflow of water, and assess the soils and products before any harvesting if contamination sources have been identified in the general vicinity of crop production areas. The land-use assessment should clearly document your sewage treatment/septic system, identify nearby municipal/commercial sewage treatment facilities, and verify that the system on your property is functioning properly and does not pose a contamination risk.
1-23: A previous land-use risk assessment has been performed. (Yes, No, R)
This question requires the auditor to review the previous land-use history with you.
1-24: When previous land-use history indicates a possibility of contamination, preventative measures have been taken to mitigate the known risks and soils have been tested for contaminants and the land-use is commensurate with test results. (Yes, No, N/A, R)
This question requires the auditor to look for evidence of old building sites or other risk factors while conducting the onsite review. For example, recent land history may indicate previous use as a dairy, feedlot or other waste site. The auditor will review soil test results for microbial contamination and any adjustments you have made to land use for crops that have minimal contact with soil. The auditor will mark the question as N/A if the land-use history assessment does not indicate a possibility of contamination.
1-25: Crop production areas that have been subjected to flooding are tested for potential microbial hazards. (Yes, No, N/A, R)
This question requires the auditor to look for evidence of flooding or locations where flooding could easily occur, and requires the auditor to ask you if/when flooding last occurred. To determine the suitability of field use, test property previously flooded for harmful pathogens prior to planting crops. The auditor will look for your test results and evaluate the potential contamination risks of crops being planted and harvested in the current (annual) or subsequent (perennial) seasons. The auditor will mark this question as N/A if no flooding has occurred on your farm.
Traceability (Item 1-26)
1-26: Each production area is identified or coded to enable traceability in the event of a recall. (Yes, No, N/A, R)
This question requires the auditor to review your map or records to verify that you have documentation that shows the crops grown in each field or production area, and that your documentation supports product traceability one step forward and backward within the marketing chain.
Field Harvest and Field Packing Activities
Within the Field Harvest and Field Packing Activities scope, 21 questions cover three main topic areas: (a) preharvest assessment (Item 2-1), (b) field sanitation units (2-2 through 2-5) and (c) field harvesting and transportation (2-6 through 2-21). The emphasis of this scope pertains to harvesting activities and the containers and equipment used to pack the product(s). The auditor may only conduct this scope when commodities are being harvested in the crop production areas covered by the audit. While the auditor is not required to observe every commodity listed on the audit, your food safety plan must address all commodities and present risks associated with different harvesting methods (e.g., mechanically harvested vs. hand harvested). You must maintain traceability records for all commodities being harvested.
Preharvest Assessment (Item 2-1)
2-1: A documented preharvest assessment is made on the crop production areas. Risks and possible sources of crop contamination are noted and assessed. (Yes, No, D)
Your operation must have a written and completed preharvest assessment for each production area prior to harvesting any crop(s) being certified by the audit. The auditor will look for your assessment to address known risks that are applicable to your operation.
Field Sanitation Units (Items 2-2 through 2-5)
2-2: The number, condition and placement of field sanitation units comply with applicable state and/or federal regulations. (Yes, No, N/A)
This question requires the auditor to verify that (a) you have the appropriate ratio of toilet facilities to the number of workers, (b) your facilities are clean and accessible to the workers, (c) the facilities are properly located in relation to irrigation or other water sources and (d) facilities are well supplied with paper, soap and waste disposal devices. The auditor will determine whether sewage from portable toilet facilities has been properly disposed of and that the facilities are serviced regularly. The auditor may only mark this question as N/A when field sanitation units are not required of your operation.
2-3: When question 2-2 is answered N/A (sanitation units are not required), a toilet facility is readily available for all workers. (Yes, No, N/A)
When the size of your operation is small and onsite sanitation units are not required, you must still provide a toilet facility and handwashing station to all workers. In this case, the auditor may observe your home toilet facilities that you have made available to all workers. This question can only be marked N/A if question 2-2 has been marked “Yes.”
2-4: Field sanitation units are in a location that minimizes the potential risk for product contamination and are directly accessible for servicing. (Yes, No, N/A)
This question requires the auditor to review the accessibility of your field sanitation unit location(s). The auditor will mark the question as “No” for locations that are inaccessible, such as situated on a narrow roadway with no easy access for servicing. This question may be marked with an N/A when field sanitation units or toilet facilities are not present.
2-5: A response plan is in place for the event of a major spill or leak of field sanitation units or toilet facilities. (Yes, No, N/A, P)
This question requires the auditor to review your policies and verify that you have written emergency cleanup procedures should contamination occur. The auditor will look for your procedures to clearly define how you will contain any spills, prevent further contamination, clean up any spills and what you will do with contaminated product(s). This question may be marked with an N/A when field sanitation units or toilet facilities are not present.
Field Harvesting and Transportation (Items 2-6 through 2-20)
2-6: All harvesting containers and bulk hauling vehicles that come in direct contact with product are cleaned and/or sanitized on a scheduled basis and kept as clean as practicable. (Yes, No, D)
You are required to keep harvest containers (picking buckets, baskets, bulk bins, etc.) as clean as possible to prevent cross-contamination of fresh produce. The auditor will review your documentation to see that your operation is adhering to this policy and that you have an individual in charge of your harvesting equipment. The auditor will observe and verify that the individual knows how the equipment is being used, that it is functioning properly, and that steps are being taken to properly clean and sanitize the equipment when needed. The auditor will crosscheck your policies and records with his/her observations to make sure that:
- Harvest containers that are used repeatedly have been cleaned after each load is delivered and prior to reuse.
- Containers stored outside are cleaned and sanitized before being used to haul fresh produce.
- Workers do not stand in bins.
- Bulk hauling vehicles are swept out on a regular basis after carrying unwashed crops.
- Food harvest containers are never used to store hazardous chemicals.
- Reused containers are clean and free of debris.
- Dirty packing containers that cannot be cleaned prior to use do not come in contact with food.
- Containers, such as picking bins, are not used for picking and transporting produce if they have been used (and marked) as refuse receptacles.
- Final shipping containers are clean at time of harvest.
2-7: All hand-harvesting equipment and implements (knives, pruners, machetes, etc.) are kept as clean as practical and are disinfected on a scheduled basis. (Yes, No, N/A, D)
This question requires the auditor to determine whether or not you have a policy for cleaning equipment and harvesting aids. Your food safety manual must show the cleaning and disinfecting schedules for implements. The auditor will question workers about the cleaning processes and crosscheck their practices with your written policies and records. This question only can be answered as N/A if your operation does not use hand-harvesting implements during harvest activities.
2-8: Damaged containers are properly repaired and disposed of. (Yes, No, N/A)
The auditor will examine your harvesting containers, such bins, packing containers and the bulk container part of vehicles, to evaluate how your operation handles the repair or disposal of damaged containers. Damaged harvesting containers should not be used, and the auditor will look for your regular inspection logs of container inventory, including containers that are properly repaired and those that are damaged and properly disposed.
2-9: Harvesting equipment and/or machinery that comes in contact with produce is in good repair. (Yes, No, N/A)
This question requires the auditor to verify that your field equipment/machinery is not leaking fluids and is free from loose or damaged parts that can be a source of physical contamination. This question may be answered N/A if you have no equipment/machinery that comes in contact with product during harvest.
2-10: Light bulbs and glass on harvesting equipment are protected so as not to contaminate produce or fields in the case of breakage. (Yes, No, N/A)
This question applies to mechanical harvesters used for root crops or machinery that sits directly over the unharvested crop. Examples of protection include plastic or wire covers, or enclosed fixtures. The auditor may answer this question as N/A if you have no glass on the harvesting equipment.
2-11: There is a standard operating procedure or instructions on what measures should be taken in the case of glass/plastic breakage and possible contamination during harvesting operations. (Yes, No, N/A, P)
This question requires the auditor to verify that your food safety plan includes documented instructions for staff to follow when glass/plastic breakage occurs and poses potential product contamination, especially for crops that are mechanically harvested.
2-12: There is a standard operating procedure or instructions on what measures should be taken in the case of product contamination by chemicals, petroleum, pesticides or other contaminating factors. (Yes, No, N/A, P)
This question requires the auditor to verify that your food safety plan outlines documented instructions for staff to follow when product becomes contaminated by chemicals, petroleum or pesticides.
2-13: For mechanically harvested product, measures are taken during harvest to inspect for and remove foreign objects such as glass, metal, rocks or other dangerous/toxic items. (Yes, No, N/A)
The auditor will observe harvesting processes or interview harvesters to determine the extent that products are being inspected continuously for the presence of nonproduce contaminants.
2-14: Harvesting containers, totes, etc. are not used for carrying or storing nonproduce items during the harvest season; farm workers are instructed in this policy. (Yes, No, P)
This question requires the auditor to examine your policy on the proper use of harvesting containers. The auditor will verify this policy by questioning workers and observing their practices. This question will be answered “No” if the auditor finds that any equipment that is being used to haul garbage, manure or other potentially contaminating items also is being used to hold fresh produce.
2-15: Water applied to harvested product is microbially safe. (Yes, No, N/A, R)
This question requires the auditor to review your water test results to determine that your water is safe from microbial contamination. The auditor may mark this question as N/A when water is not applied to your field-harvested product. (See the water use discussion in the Farm Review Section of this manual, p. 41-42).
2-16: Efforts have been made to remove excessive dirt and mud from product and/or containers during harvest. (Yes, No, N/A)
This question requires the auditor to examine your crops and harvest containers, and verify that you take efforts to remove excess dirt/mud to prevent the spread of potential contamination to other areas of the farming operation.
2-17: Transportation equipment used to move product from field to storage areas or storage areas to processing plant that comes into contact with product is clean and in good repair. (Yes, No, N/A)
This question requires the auditor to examine the cleanliness of your transportation equipment that is used to move product. If using an open truckbed to haul unprotected produce (e.g., large melons or pumpkins), line it, cover the food with clean washable covers and make sure the transportation vehicle is clean. The auditor will review records and documentation (e.g., logs) to verify that your policy for regularly scheduled equipment cleaning is timely and followed.
2-18: There is a policy in place and has been implemented that harvested product being moved from field to storage areas or processing plants is covered during transportation. (Yes, No, P)
This question requires the auditor to examine the measures you take to protect product(s) during transportation. When transporting product(s) in bulk, such as from the field or from storage facilities, take steps to reduce possible contamination by other vehicles on the roads, overhead contamination from overpasses, birds, etc. Examples of protection include the use of tarps and enclosed trailers to transport products. If you move products in enclosed containers, such as boxes and cartons, the auditor will not consider the products to be protected and covered during transportation. The auditor will question your staff to verify that produce is consistently covered across loads when transported from the field to the packing operation. The auditor will observe loads being delivered to validate the responses of your staff.
2-19: In ranch or field pack operations, only new or sanitized containers are used for packing the product. (Yes, No, N/A, D)
This question requires the auditor to verify your policies and practices for using new or sanitized containers when packing product. While the use of new containers eliminates the possibility of cross-contamination from used containers, you may find it necessary to pack produce into reusable plastic containers (RPC) to meet buyer specifications. If you are using RPCs, the auditor will observe that these containers are sanitized prior to each reuse in the field. Additionally, the auditor will review cleaning logs or records to verify that the containers have been sanitized. This question can be marked with N/A if your operation does not do any field packing.
2-20: Packaging materials used in ranch or field pack operations are properly stored and protected from contamination. (Yes, No, N/A)
This question requires the auditor to verify where and how you store packing containers. Store containers in a manner that keeps them free of contamination from potential sources of pests (such as rodents), dirt and water. When stored outside, cover and protect all containers from the elements (rain, wind), bird droppings, etc. It is not sufficient to use the top container as the cover to protect the pile below. The auditor will be required to answer this question as “No” if containers are stored in a manner that poses a contamination risk.
Traceability (Item 2-21)
2-21: Product moving out of the field is uniquely identified to enable traceability in the event of a recall. (Yes, No, D)
This question requires the auditor to examine your policy on marking and tracking products, and to verify that you have a system in place to quickly and efficiently track product(s) one step forward and backward in the food chain. As examples, identification records for products moving in bulk from the field to a packing house or storage facility may include records of load tickets, field harvest records attached to the load or similar records that show where the product originated. For products that are field packed, the auditor will look for individual containers (or master containers) to include company information such as the name, address or other identifying marks that you have outlined in your traceability program. (See the traceability discussion in the General Questions section of this manual, p. 35-36.)
Conclusion
Maintaining safe food is of paramount importance for growers and consumers. This manual provided technical and state resources to help increase food safety on the farm for specialty crop growers, focusing on fruit and vegetable crops. It provided several examples and tips, along with state and national websites, to help you improve good agricultural practices in your operation.
As you worked your way through this manual, we highlighted the benefits of achieving USDA GAP&GHP certification and what to anticipate during the audit process. The audit checklists in the manual can be ongoing resources as you identify areas in your operation that may present food-related risks, whether or not you decide to seek certification.
If you are just getting started creating a food safety plan for your operation, our suggestion is to start small and identify a few processes on your farm that you can easily modify. Once you have successfully made those changes, you can work on more complex processes. We hope this manual helps you move forward with developing and implementing safety programs for your operation to help ensure a safe food supply.
Footnotes
- The AMS offers voluntary, user-fee funded programs to support strategic marketing of agricultural products.
- This definition was put forward by Section 101 of the Specialty Crops Competitiveness Act of 2004 (7 U.S.C. 1621 note) and amended under Section 10010 of the Agricultural Act of 2014, Public Law 113-79 (the Farm Bill). For a list of eligible commodities, see https://www.ams.usda.gov/services/grants/scbgp/specialty-crop.
- The FDA regulates fresh fruits and vegetables.
References and Resources
- N.D. Department of Agriculture (n.d.). Home. https://www.nd.gov/ndda/
- North Dakota State University (n.d.) Field to Fork. www.ag.ndsu.edu/fieldtofork
- USDA Agricultural Marketing Service (n.d.) Form SC-237A – Request for Audit Services.
https://www.ams.usda.gov/resources/sc237a - USDA Agricultural Marketing Service (2008). Fresh Products Branch Directive: Fresh Products Branch Good Agricultural Practices and Good Handling Practices (GAP&GHP) Audit Appeals, Complaints and Dispute Procedures. https://www.ams.usda.gov/sites/default/files/media/FPB-703%20%E2%80%93%20Fresh%20Products%20Branch%20GAP-GHP%20Audit%20Procedures.pdf
- USDA Agricultural Marketing Service (n.d.) Good Agricultural Practices (GAP) & Good Handling Practices (GHP). https://www.ams.usda.gov/services/auditing/gap-ghp
- USDA Agricultural Marketing Service (n.d.) (November 2009) Good Agricultural Practices Good Handling Practices Audit Verification Program: Policy and Instructions. https://www.ams.usda.gov/sites/default/files/media/GAP-GHP%20Audit%20Verification%20Program%20Policies%20and%20Procedures_0.pdf
- USDA Agricultural Marketing Service (n.d.) (April 2011). Good Agricultural Practices and Good Handling Practices Audit Verification: User’s Guide.
https://www.ams.usda.gov/sites/default/files/media/GAPGHP_Audit_Program_User%27s_Guide%5B1%5D.pdf - U.S. Food and Drug Administration (1998). Guidance for Industry: Guide to Minimize Microbial Food Safety Hazards for Fresh Fruit and Vegetables. https://www.fda.gov/downloads/Food/GuidanceComplianceRegulatoryInformation/GuidanceDocuments/ProduceandPlanProducts/UCM169112.pdf
- USDA Agricultural Marketing Service (n.d.) How to Apply for Auditing Service. https://www.ams.usda.gov/services/auditing/apply
- USDA Agricultural Marketing Service (n.d.) Specialty Crops. https://www.ams.usda.gov/market-news/fruits-vegetables
- USDA Agricultural Marketing Service (n.d.) Uploading USDA Audit Reports. https://www.ams.usda.gov/services/auditing/gap-ghp/audit-upload-options
- USDA Agricultural Marketing Service (n.d.) USDA definitions of specialty crop. https://www.ams.usda.gov/sites/default/files/media/USDASpecialtyCropDefinition.pdf
- USDA Agricultural Marketing Service (n.d.) USDA GAP&GHP Audit. https://www.ams.usda.gov/services/auditing/gap-ghp/audit
- USDA Agricultural Marketing Service (September 2014). USDA Good Agricultural Practices Good Handling Practices Audit Verification Checklist. https://www.ams.usda.gov/sites/default/files/media/GAPGHP_Checklist_no_spell_Checklist_Enabled%5B1%5D.pdf
- USDA Agricultural Marketing Service (April 2011). USDA Good Agricultural Practices Good Handling Practices Audit Verification Program: User’s Guide for GAP&GHP. https://www.ams.usda.gov/sites/default/files/media/GAPGHP_Audit_Program_User%27s_Guide%5B1%5D.pdf
- USDA Agricultural Marketing Service (n.d.) USDA Market News. https://www.ams.usda.gov/market-news
- USDA Agricultural Marketing Service (n.d.) SC430 - Vendor Form. https://www.ams.usda.gov/resources/sc430
- USDA Agricultural Marketing Service (n.d.) SC-651 – Agreement for Participation in Audit Verification Programs. https://www.ams.usda.gov/resources/sc651
- USDA Agricultural Marketing Service (n.d.) Service Fees. https://www.ams.usda.gov/services/grading/fees
- USDA Agricultural Marketing Service (n.d.) Website posting of companies with acceptable audits: Instructions
for use based on report type. https://www.ams.usda.gov/sites/default/files/media/GAPGHPSiteInstructions%5B1%5D.pdf
This project was supported by the U.S. Department of Agriculture's (USDA) Agricultural Marketing Service through grant 16-SCBGPND-0029. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the USDA.

