Phosphorus Behavior In the Environment (NM1298, Revised June 2018)
Availability: Web only

Photo: iStock.com
Phosphorus (P) is a naturally occurring element that exists in minerals, soil, living organisms and water. Plant growth and development require phosphorus, like nitrogen, in large amounts. Phosphorus is essential for early root development and hastens plant maturity.
The forms of phosphorus present in soil can include organic, soluble or “bound” forms. Understanding the relationship among these forms of phosphorus is necessary to understand plants’ utilization of phosphorus and the extent to which phosphorus can move within the environment. Note that phosphorus is the least mobile of the major plant nutrients.
■ Organic phosphorus — a part of all living organisms, including microbial tissues and plant residue. It is the principal form of phosphorus in the manure of most animals. About two-thirds of the phosphorus in fresh manure is in the organic form.
■ Soluble phosphorus — sometimes called available inorganic phosphorus. It can include small amounts of organic phosphorus, as well as orthophosphate, the form taken up by plants. It also is the form subject to loss by dissolution in runoff and, to a lesser extent, leaching.
The soluble form accounts for the smallest proportion of the total phosphorus in most soils. When fertilizer or manure (both containing mostly soluble phosphorus) is added to soil, the soil’s pool of soluble phosphorus increases. With time, soluble phosphorus is transformed slowly to less-soluble (less plant-available) forms.
■ Attached or “bound” phosphorus — unavailable inorganic phosphorus. A large amount of the soil’s phosphorus is bound in compounds that are formed when the anionic (negatively charged) forms of dissolved phosphorus become attached to cations, such as iron, aluminum and calcium. Attached phosphorus includes “labile,” or loosely bound, and “fixed,” or tightly bound, phosphorus compounds.
Note that phosphorus loosely bound to the soil particles (labile phosphorus) remains in equilibrium with soluble phosphorus. Thus, when plant removal reduces the concentration of soluble phosphorus, labile phosphorus is converted to the soluble form to maintain the equilibrium.
Much of the phosphate that living organisms use becomes incorporated into organic compounds. When plant materials return to the soil, organic phosphate will be released slowly as available inorganic phosphate or incorporated into more stable organic materials and become part of the soil organic matter. The release of available inorganic phosphorus from organic sources is called mineralization, and microorganisms carry it out.
How Phosphorus is Lost From Agricultural Fields
Fields with high losses of phosphorus must have a high source potential and a mechanism to transport phosphorus to bodies of water. Phosphorus can travel to surface water attached to particles of soil or manure. Phosphorous also can dissolve into runoff water as it passes over the surface of the field.
Leaching of phosphorus usually is not a significant concern. Soil particles strip soluble phosphorus from the water as it moves through the soil profile. The concentration of phosphorus in soil leachate is significantly less than surface runoff concentrations.
However, special situations can produce higher concentrations of phosphorus in groundwater. The capacity of soil to absorb phosphorus can be overwhelmed on sandy soils or when the water table is close to the soil surface. Also, cracking in soils creates channels allowing surface water to travel directly to groundwater.
Phosphorus losses from agricultural fields can be divided into four categories:
■ Flash losses of soluble phosphorus soon after application of fertilizer or manure
■ Slow-leak losses of soluble phosphorus
■ Organic P released as soluble P from the rapid vegetation decomposition during spring thaw
■ Wind and/or water erosion events
Flash Losses of Soluble Phosphorus
Manure and commercial fertilizers have a vastly higher concentration of soluble phosphorus than soil. If rainfall runoff occurs soon after a surface application of manure or commercial fertilizer, the concentration of soluble phosphorus in the runoff can be more than 100 times greater than from other runoff events. Flash losses of soluble phosphorus have high concentrations of phosphorus in a form that is readily available to aquatic organisms.
Research with poultry litter and swine manure applied to pastures shows that soluble phosphorus concentrations increase in direct proportion to increasing application rates in flash phosphorus loss events. These events occur only if rainfall runoff occurs soon after a surface phosphorus application or when phosphorus is surface-applied to frozen or snow-covered fields.
Through time, highly soluble manure and fertilizer phosphorus on the soil surface will react with the soil, reducing soluble phosphorus in runoff. Normal levels return during the course of a month in warm soils, and longer in cold soils.
Manure and fertilizer on frozen or snow-covered soils is not recommended because phosphorus never has a chance to bind with soil before runoff occurs.
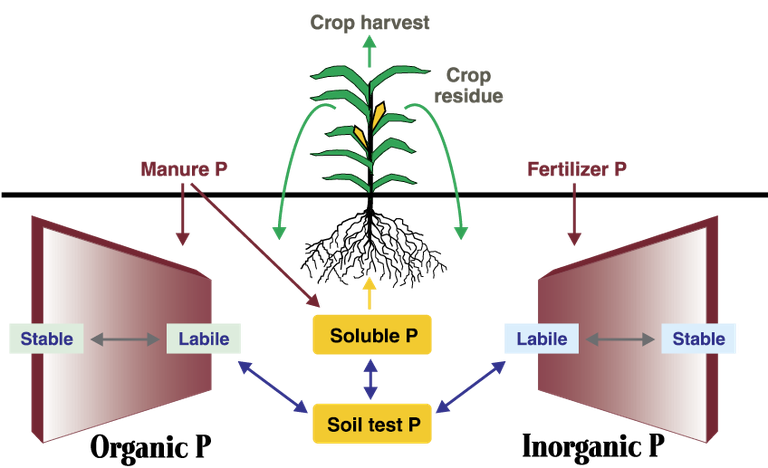
The phosphorus cycle. (Source: Livestock and Poultry Environmental Stewardship Curriculum)
Slow-leak Losses of Soluble Phosphorus
All soils release very small amounts of soluble phosphorus into surface runoff. The phosphorus level in a soil test is related to the concentration of soluble phosphorus in runoff. Substantial evidence shows soluble phosphorus concentrations in runoff increase linearly with increasing soil test phosphorus levels. However, this linear relationship varies among soil types.
Slow-leak phosphorus losses are important because they occur in every runoff event. Because of the cumulative effect of multiple runoff events, this can be an important source of phosphorus loss.
Organic P Released as Soluble P From Rapid Vegetation Decomposition During Spring Thaw
New research from Manitoba, conducted in similar soils with similar slope as in the North Dakota Red River Valley, indicates that the greatest field contribution of P into surface waters is through the release of soluble P from the rapid vegetation decomposition that happens during the spring thaw period. This research has troubling implications because the usual best management practice of establishing vegetative buffer streams (also called riparian buffer strips) between streams and fields may be the worst practice in flat topography in the northern Plains, where the main source of P in surface water bodies comes from the decomposing plants.
A possible management practice to minimize P released from riparian buffer strips may be haying the buffer strips or grazing prior to winter freeze-up. Haying or grazing minimizes the dry matter available for spring decomposition, removing P from the system, while continuing to reduce P-laden sediments from entering the water bodies.
Erosion Losses From Wind and/or Water
Phosphorus is almost entirely bound to soil particles. When soil particles move with wind and water, P moves with them. Fine soil particles have a greater capacity to hold phosphorus than coarse particles. Unfortunately, soil erosion transports more fine particles, causing the eroded sediment to be “enriched” with phosphorus.
The greatest loss of topsoil in the northern Plains is from wind erosion. An estimated 42 million tons of P in terms of P2O5 fertilizer equivalent have been lost since North Dakota first was farmed in the 1880s.
Some of this sediment was lost to water bodies such as lakes. Much of it was relocated sometimes miles away from the source. The sediment deposited by wind into local water bodies remains a perpetual P source every year and may be remedied only by dredging, as is done behind dams.
Water erosion also contributes to sediments in surface water, where the sediments contribute P to the water each season. Reducing or eliminating tillage to control erosion can reduce total phosphorus losses from wind and water greatly.
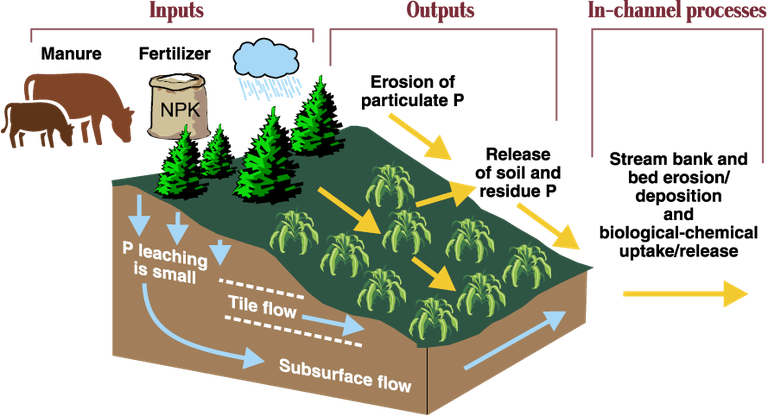
Phosphorous movement in the environment. (Source: Livestock and Poultry Environmental Stewardship Curriculum)
Managing Phosphorus in the Environment
A number of best management practices can be implemented to minimize phosphorus losses and the environmental impacts of phosphorus:
- Only apply phosphorus to fields that have an agronomic need for phosphorus.
- Incorporate surface-applied P sources below the soil surface in a manner that does not increase soil erosion when possible.
- Surface apply phosphorus sources only on fields with a low potential for runoff.
- Adopt soil conservation practices to minimize soil erosion, such as maintaining buffer strips around water resources.
- Hay or graze buffer strips in the fall to reduce the vegetation that might release P into surface water in the spring when it decomposes.
- Reduce the amount of soil lost through runoff from agricultural fields through crop selection and soil conservation practices.
For additional information on water quality, see these other NDSU Extension publications:
■ “Environmental Implications of Excess Fertilizer and Manure on Water Quality”
■ “Nitrogen Behavior in the Environment”
■ “North Dakota Fertilizer Recommendation Tables and Equations”
This publication was authored by Ron Wiederholt, NDSU Extension central district director, and Bridget Johnson, former NDSU Extension nutrient management specialist.

June 2018

