Drinking Water Quality: Testing and Interpreting Your Results (WQ1341, Revised Aug. 2019)
Availability: Web only
It’s All In Your Water
Public water systems in North Dakota cooperate with the North Dakota Department of Environmental Quality (NDDEQ) to ensure compliance with safe water guidelines set by the Environmental Protection Agency’s (EPA) Safe Drinking Water Act. These rules do not cover private wells.
The owner of a private well is responsible for testing the water, interpreting the results and making necessary changes to the system. Although the EPA cannot force private well owners to comply with the EPA guidelines, the agency’s maximum contaminant levels can serve as a reference for safe drinking water. An unacceptable water sample may be based on bacterial analysis, chemical characteristics of the water (such as chlorides, iron and hardness) or physical characteristics (such as odor, taste and color).
This publication will answer the following questions:
- What should your water be tested for?
- What samples do I need?
- Where can I have my water tested?
- How do I interpret my results?
- How do I correct my problem?
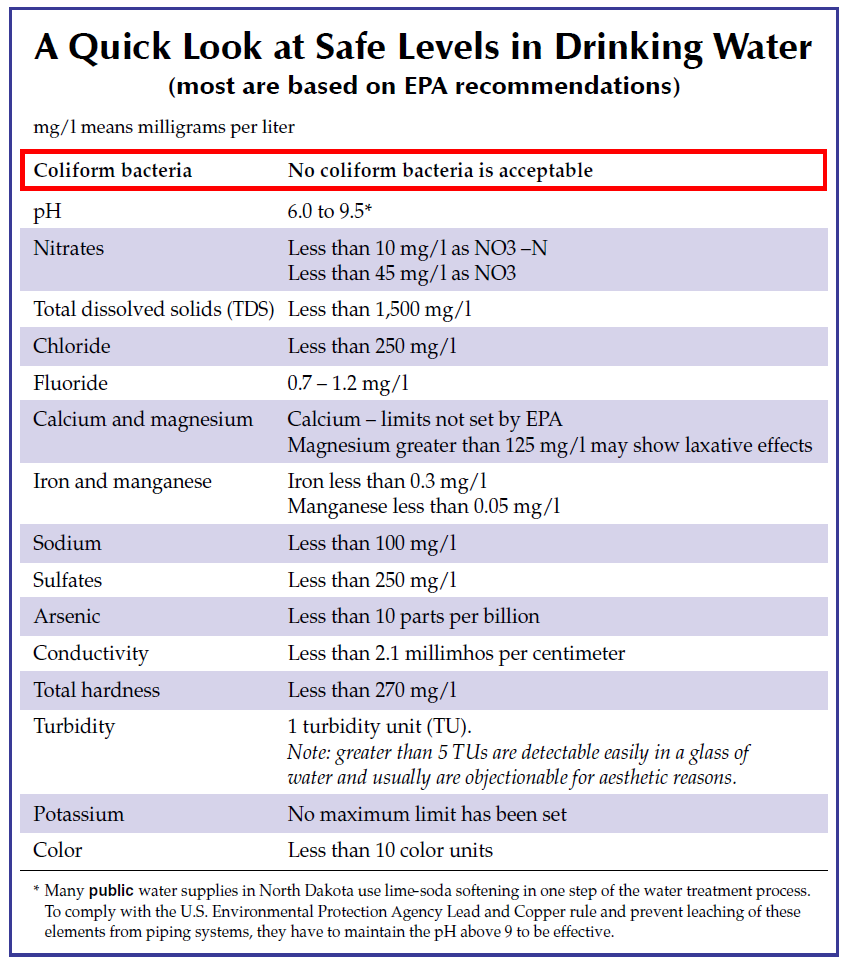
The following chart provides a quick overview of acceptable levels for drinking water. A more detailed explanation follows.
How Do I Collect a Sample?
Sample collection methods are based on the type of analysis you desire.
Bacterial Analysis
A sterile container provided by the testing laboratory is required for a bacteria test. Check with the laboratory for sampling and timing instructions because samples must reach the lab within 36 hours. Do not to rinse containers because most contain preservatives.
Routine Water Analysis for Minerals and Chemicals
A “raw” water sample is preferred for a routine water analysis. If possible, bypass water treatment units, such as water softeners, reverse osmosis (RO) systems and iron removal systems, when collecting the sample. A second sample taken after the water has passed through the treatment equipment will help you determine if your equipment is functioning properly.
Give special attention to contaminants that have tested high in the past or when concerns arise from health issues. Use a clean plastic or glass container to collect a 1-quart sample. Containers previously used for bleach, soap or other substances will contaminate the water sample. Rinse the container and lid three times with the water that will be tested. Laboratories recommend samples reach them within two weeks.
Water Sampling in Active Oil Drilling Areas
If you are concerned about water quality due to present or future oil activity, a list of suggested tests is available in NDSU publication WQ-1614, “Baseline Water Quality in Areas of Oil Activity,” or through the laboratories listed in this publication.
Where Do I Have My Water Tested?
A list of laboratories in North Dakota can be found on the last page of this publication, on the internet at www.ndsu.edu/waterquality, at your local Extension office or at the North Dakota Department of Environmental Quality at 701-328-6140. To select a lab, consider convenience and services offered.
What Should My Water Be Tested For?
New wells or homes:
- Bacteria
- Routine water analysis, including:
– Conductivity
– Magnesium
– Manganese (total)
– Sodium absorption ratio (SAR)
– pH
– Sodium
– Nitrates
– Total dissolved solids (TDS)
– Calcium
– Iron (total)
– Hardness
Existing wells: Annual testing
- Each year, general indicators, including:
– Bacteria, pH, nitrate and total dissolved solids
– Any constituents that were at or near the drinking water standard in previous years
Existing wells: Every five years or if you notice a change in water quality
- Comprehensive water analysis
- Routine water analysis, plus:
– Potassium
– Alkalinity
– Chloride
– Fluoride
– Sulfate
Note: Keep copies of all results so you can track changes in your water quality through time.
Now That I Have the Results, What Do These Numbers Mean?
Figures 1 and 2 are examples of water analyses reports. The report will contain a list of contaminants for which the water was tested and the measured concentration of each. The report also may highlight any problems.
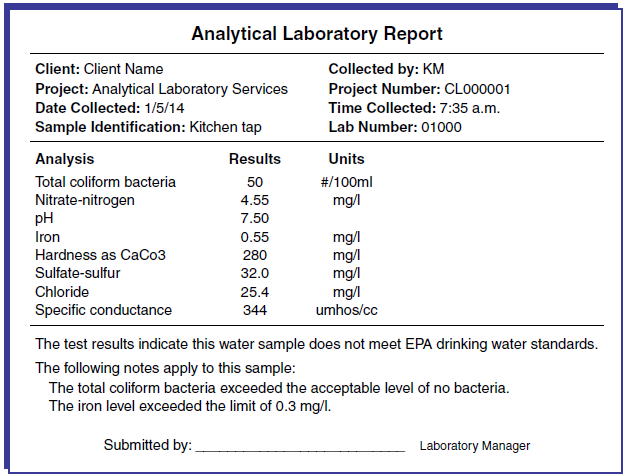
Figure 1. Sample Analytical Laboratory Report
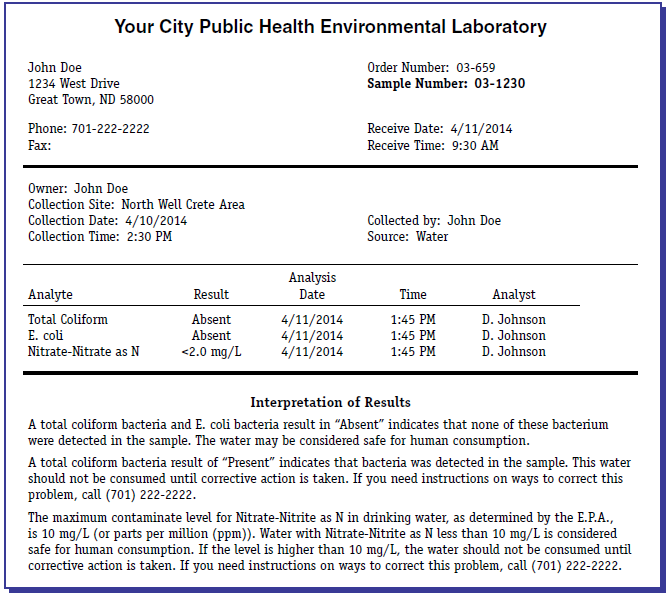
Figure 2. Sample Bacteriological Testing Report
The concentration is the amount of a given substance (weight) in a specific amount of water (volume). The most common concentration unit used is milligrams per liter (mg/l), which, in water, is approximately equal to one part per million (ppm).
Many compounds are measured in smaller concentrations, such as micrograms per liter or parts per billion (ppb). Some contaminants have units that are specific to the test and others are expressed as an index number and not in terms of concentration, and therefore have no units.
An online water quality interpretation tool has been developed to assist you in evaluating your drinking, livestock and irrigation water quality test reports. A link to the interpretive tool can be found at: https://erams.com/wqtool.
Instructions on how to use the interpretive tool are on the website. After you enter the numbers from your water test report, the tool will provide guidelines for acceptable or unacceptable concentrations.
For more information:
- U.S. Environmental Protection Agency, Safe Drinking Water Act
- North Dakota Department of Environmental Quality
Interpreting a Bacteriological Test
All water has some form of bacteria in it. The presence of bacteria does not mean the water is unsafe to drink. Only disease-causing bacteria known as pathogens lead to disease. Your test results should include total coliform bacteria. Total coliform bacteria are a group of several kinds of bacteria commonly found in the environment, including soil, vegetation and untreated surface water. They also are found in the intestinal tract of warm-blooded animals, including humans.
A laboratory commonly will report the bacteriological test as positive or negative, indicating the presence or absence of total coliform bacteria. A negative total coliform bacteria result means the water is safe for human consumption from a bacteriological standpoint.
A positive total coliform test would indicate unsanitary conditions and the possible presence of disease-causing organisms. Further testing should include the subgroup fecal coliform and its subgroup, Escherichia coli (E. coli). A positive fecal coliform would indicate possible recent sewage or animal waste contamination.
E.coli outbreaks related to food contamination have received media attention. These outbreaks are caused by a specific strain of E. coli known as E. coli 0157:H7. A positive E. coli result does not necessarily mean this specific strain is present. However, it does indicate recent fecal contamination, which should be interpreted as an indication of a greater risk that pathogens are present.
Disease-causing microbes (pathogens) in these wastes can cause diarrhea, cramps, nausea, headaches or other symptoms. These pathogens may pose a special health risk for infants, young children and people with severely compromised immune systems.
Shock chlorination should be performed on a well that reports a positive E.coli or fecal coliform test. For instructions on chlorination, watch this Shock Chlorination of a Private Well video.
Repeat the bacteria test within seven days to confirm the effectiveness of the chlorination.
Interpreting a Mineral Analysis
Alkalinity
Alkalinity is a measure of the capacity of water to neutralize acids. The predominant chemicals present in natural waters are carbonates, bicarbonates and hydroxides. The bicarbonate ion is usually prevalent. However, the ratio of these ions is a function of pH, mineral composition, temperature and ionic strength. Water may have a low alkalinity rating but a relatively high pH or vice versa, so alkalinity alone is not of major importance as a measure of water quality.
Alkalinity is not considered detrimental to humans but generally is associated with high pH values, hardness and excessive dissolved solids. High-alkalinity waters also may have a distinctly flat, unpleasant taste. Treatment is an ion exchange via the addition of a tank media or reverse osmosis.
Arsenic
Arsenic is a semimetalic element that is odorless and tasteless. It enters drinking water supplies from natural deposits in the earth, or from agricultural and industrial practices.
According to the EPA, long-term exposure to arsenic in drinking water is linked to cancer of the bladder, lungs, skin, kidneys, nasal passages, liver and prostate. Noncancerous effects of ingesting arsenic include cardiovascular, pulmonary, immunological, neurological and endocrinal (for example, diabetes) problems.
Treatment depends on the level of contamination. Typical recommendations include the addition of an anion filter or tank media.
Refer to the list of publications at the end of this page more information on filtration.
Calcium and Magnesium
Calcium and magnesium are the main contributors to water hardness. When water is heated, calcium breaks down and precipitates out of the solution, forming scale. Maximum limits have not been established for calcium. Magnesium concentrations greater than 125 mg/l may have a laxative effect on some people. Treatment for calcium is softening (tank media) and reverse osmosis. Magnesium levels can be controlled through distillation.
Chloride
High concentrations of chloride ions can cause water to have an objectionable salty taste and corrode hot-water plumbing systems. High-chloride waters have a laxative effect for some people. An upper limit of 250 mg/l has been set for chloride ions, although noticing the taste at this level is difficult, and even higher concentrations do not appear to cause adverse health effects. An increase in the normal chloride content of water may indicate possible pollution from human sewage, animal manure or
industrial wastes.
Color
Color may indicate dissolved organic material, inadequate treatment and high disinfectant demand, and may indicate the potential for the production of excessive amounts of disinfectant byproducts. Inorganic contaminants, such as metals, are also common causes of color. In general, the point of consumer complaint is variable, ranging from 5 to 30 color units, although most people find color objectionable in excess of 10 color units. Other contaminants that may be related to change in water color include aluminum, copper, foaming agents, iron, manganese and total dissolved solids. Treatment is reverse osmosis.
Conductivity
Conductivity is a measure of the conductance of an electric current in water. This is an easy measurement to make and relates closely to the total dissolved solids (mineral) content of water. The maximum contaminant level (MCL) is 0.4 to 0.85 micro Siemens per centimeter. Treatment with reverse osmosis is effective for drinking water purposes.
Fluoride
Fluoride concentrations of 0.7 to 1.2 mg/l in drinking water will protect against dental cavities. However, excessive levels (more than 1.5 mg/l) may cause discoloration, or mottling of the teeth. This occurs only in developing teeth before they push through. Elevated fluoride levels also may cause skeletal damage and bone disease. Because low levels of fluoride are common in groundwater, most municipalities add fluoride to the water.
Iron and Manganese
Iron in concentrations greater than 0.3 mg/l and manganese in concentrations greater than 0.05 mg/l may cause brown and black stains on laundry, plumbing fixtures and sinks. A metallic taste also may be present, and it may affect the taste of beverages made from the water. High concentrations of iron and manganese do not appear to present a health hazard. Treatment includes a water softener or iron filter for iron and reverse osmosis for manganese.
Refer to the list of publications at the end of this page for more information on softening, and iron and manganese removal.
Nitrates
The results reported for nitrates can be confusing because they may be reported as nitrogen (N) or nitrate-nitrogen or as nitrate (NO3). The following are the maximum levels for each:
- Nitrogen (N) or nitrate-nitrogen (NO3-N) should not be higher than 10mg/L.
- Nitrate (NO3) should not be higher than 45mg/L.
High nitrate levels may cause methemoglobanemia (infant cyanosis or “blue baby disease”) in infants who drink water or formula made from water containing nitrate levels higher than recommended.
Adults can drink water with considerably higher concentrations than infants without adverse effects. Treatment of such water includes anionic ion exchange, reverse osmosis, distillation and/or deionization.
Refer to the list of publications at the end of this page for more information on softening.
pH
The pH of water is a measure of acidity or alkalinity. The pH is a logarithmic scale based on a measure of the free hydrogen ions in the water. The scale runs from 0 to 14, where 7 is considered neutral, 0 to 7 is acidic and 7 to 14 is alkaline. Because pH can be affected by dissolved minerals and chemicals, it is an important indicator of the change in water chemistry.
According to the U.S. Environmental Protection Agency, drinking water with a pH between 6.0 and 9.5 generally is considered satisfactory. Several public water supplies that use the Missouri, James or Red River as their source of water have to maintain the pH above 9 keep them in compliance with the Lead and Copper rule of the Safe Drinking Water Act, which details how to prevent leaching of these elements from piping systems.
Water with a pH below 6 or above 9.5 can be corrosive to metal plumbing pipes and fixtures. The pH of water can affect the performance of pesticides, particularly herbicides.
Potassium
Potassium concentrations in water are generally very small. Although excessive amounts may have a laxative effect, the EPA has not established a maximum limit. Potassium (chloride) is used as a replacement for salt in water softeners when dietary sodium intake is a health issue.
Sodium
Sodium is a very active metal that does not occur naturally in a free state. It always is combined with other substances. In the human body, sodium helps maintain the water balance. Human intake of sodium is mainly influenced by the consumption of sodium as sodium chloride or table salt. The contribution of drinking water is normally small, compared with other sources.
The treatment for certain heart conditions, circulatory or kidney diseases, or cirrhosis of the liver may include sodium restriction. Diets for these people should be designed with the sodium content of their drinking water taken into account.
The National Academy of Sciences has suggested a standard for public water allowing no more than 100 mg/l of sodium. This would ensure that the water supply adds no more than 10 percent of the average person’s total sodium intake.
The American Health Association recommends a more conservative standard of 20 mg/l to protect heart and kidney patients.
Softening by ion exchange or lime-soda ash increases the sodium content approximately 8 mg/l for each gr/gal (grain per gallon) of hardness removed. Treatment includes the use of potassium chloride instead of sodium chloride softener pellets (softener salt) or, alternatively, restricting drinking water from this source.
Sulfates
Water containing high levels of sulfates, particularly magnesium sulfate (Epson salts) and sodium sulfates (Glauber’s salt) may have a laxative effect on people unaccustomed to the water. These effects vary among individuals and appear to last only until they become accustomed to using the water. High sulfate content also affects the taste of water and forms a hard scale in boilers and heat exchangers. The upper limit recommended for sulfates is 250 mg/l. Treatment includes reverse osmosis.
Total Dissolved Solids (TDS)
High concentrations of TDS may affect taste adversely and deteriorate plumbing and appliances. The EPA recommends that water containing more than 500 mg/l of dissolved solids not be used if other less mineralized supplies are available. However, water containing more than 500 mg/l of TDS is not dangerous to drink. Exclusive of most treated public water supplies, the Missouri River, a few freshwater lakes and scattered wells, very few water supplies in North Dakota contain less than the recommended 500mg/L concentration of total dissolved solids. Many households in the state use drinking water supplies with concentrations up to 2,000 mg/l and greater. Treatment for household use is reverse osmosis.
Total Hardness
Hardness is the property that makes water form an insoluble curd with soap and primarily is due to the presence of calcium and magnesium. Very hard waters have no known adverse health effects and may be more palatable than soft waters. Hard water is primarily of concern because it requires more soap for effective cleaning; forms scum and curd; causes yellowing of fabrics; toughens vegetables cooked in the water; and forms scale in boilers, water heaters, pipes and cooking utensils. The hardness of high-quality water should not exceed 270 mg/l (15.5 grains per gallon) measured as calcium carbonate. Water softer than
30 to 50 mg/l may be corrosive to piping, depending on pH, alkalinity and dissolved oxygen. Water softeners will correct hard water of more than 270 mg/l.
Refer to the list of publications at the end of this page for more information on softening.
Turbidity
Turbidity is a measure of suspended minerals, bacteria, plankton, and dissolved organic and inorganic substances. Turbidity often is associated with surface water sources. Treatment includes mixing with a substance such as alum that causes coagulation of the suspended materials, which then can be removed by sand filter filtration.
Water Testing Labs in North Dakota
The following chart lists laboratories in North Dakota that test drinking water.
This publication was authored by Roxanne Johnson, former water quality associate
Related Publications
WQ1029 Filtration: Sediment, Activated Carbon and Mixed Media
WQ1030 It’s All in Your Water: Iron and Manganese Removal
WQ1031 Water Softening (Ion Exchange)
WQ1352 What‘s Wrong With My Water? Choosing the Right Test
WQ1614 Baseline Water Quality in Areas of Oil Development
The printing and development cost of this publication was paid, in part, by the Northern Plains and Mountains Regional Water Program in partnership with the USDA-NIFA.
NDSU Extension is solely responsible for the content of this publication.
This material is based upon work supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, under Agreement No. 2004-51130-022848

