What is Soil Acidity? (SF2012, March 2021)
Soil acidity is a condition in which the soil pH is lower than a neutral pH (less than 7). Soil pH is a measure of the hydrogen (H+) ion concentration expressed as the negative common logarithm of H+ concentration. It is an index of the activity of H+ as it interacts with soil components, nutrients in the soil solution (water) and plants growing in the soil. Figure 1 shows the pH scale and its interpretation in soils.
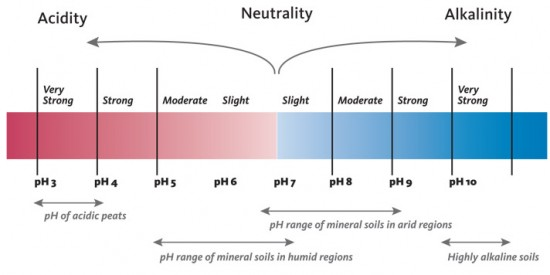
- Figure 1. The range in pH normally found in soils under native or natural conditions. pH values below 7 indicate greater soil acidity as values become lower. (Adapted from Landscape for Life, University of Texas, Austin)
Causes of Soil Acidity
Initial soil characteristics such as soil parent material, climate and original native vegetation determined soil pH prior to their cultivation. Some North Dakota soils were formed on acidic parent materials and are naturally slightly acidic. Repeated use of ammonium-based acid-forming fertilizers, leaching of nitrate-N (NO3-N) and plants taking up cations and leaving anions all promote acidification of the topsoil when cultivated.
Well-drained sandy soils in North Dakota and soils in which kaolinite is the predominant clay mineral, often in the unglaciated southwestern portions of the state, are the most susceptible to becoming more acidic. Table 1 illustrates the amount of acidity formed by common ammonium-N containing fertilizer materials when the ammonium-N is converted to nitrate-N and the lime equivalent (100% CaCO3) required to neutralize the resulting acidity.
Table 1. Lime quantity required to neutralize the soil acidity produced by different N sources if all of the ammonium-N is converted to nitrate-N.
| Nitrogen Source | Fertilizer Analysis |
Lime Required |
| Anhydrous ammonia | 82-0-0 | 1.8 |
| Urea | 46-0-0 | 1.8 |
| Ammonium nitrate | 34-0-0 | 1.8 |
| Ammonium sulfate | 21-0-0-24 | 5.4* |
| Monoammonium phosphate | 11-52-0 | 5.4 |
| Diammonium phosphate | 18-46-0 | 3.6 |
| Urea-ammonium nitrate solutions | 28 to 32-0-0 | 1.8 |
From Wortmann et al. (2015) as adapted from Havlin et al., 2005.
*The estimate for ammonium sulfate may be 50% too high (Chien et al., 2010)
Types of Soil Acidity
Two types of acidity occur in soils. Soil acidity determined by pH measurement during a routine soil test is known as active acidity. This is the concentration of H+ ions in the soil solution when measured in a 1:1 soil-to-water ratio mixture.
However, not all H+ ions are released immediately by the soil into solution. A portion of the H+ ions remain attached to negatively charged exchange sites on clay and organic matter (OM) particles.
This acidity is called reserve acidity because H+ can be released into solution as soil solution conditions change due to moisture changes, and concentrations of dissolved ions and salts occur. This acidity can be measured by the addition of a dilute calcium chloride solution (0.01 M CaCl2) or a buffer to the water pH suspension.
Active and reserve acidity (measured as water and buffer pH) are related but their relationship varies due to types and quantities of clay minerals, organic matter and free lime in the soil. Soil cation exchange capacity (CEC), which is related to the type and quantity of clay and organic matter, influences the ratio of reserve acidity to active acidity. Soils with a higher CEC (higher clay and OM) resist (buffer) acidification better than soils with a low CEC (sandy, lower clay and OM).
Effect of Soil Acidity on Plant Nutrient Availability
Soil acidity affects nutrient availability and microbial activity as well as plant growth. Figure 2 shows the relationships between soil pH and plant nutrient availability.
Each nutrient is represented by a band indicating its availability across the normal pH range of soils. When the band is narrow, a nutrient is relatively unavailable, while a wide band indicates a high availability.
Plant nutrients such as nitrogen (N), phosphorus (P), potassium (K), sulfur (S), calcium (Ca), magnesium (Mg) and molybdenum (Mo) have low availability at strongly acidic pH values. Other nutrients such as manganese (Mn), copper (Cu) and zinc (Zn) tend to be more available until the soil gets very acid (less than pH 5). Iron (Fe) and aluminum (Al) availability increases as soil acidity increases and Al becomes toxic to plants at pH values less than 5.
Activity of N-fixing organisms such as rhizobia related to legumes decreases as soils become more acid but fungi are tolerant of soil acidity. Legume crops (alfalfa, clovers, soybeans and dry beans) are generally more sensitive to low soil pH. Activity of some herbicides is increased and decreased in others as soils become more acid, creating problems with effectiveness or carryover to following crops.
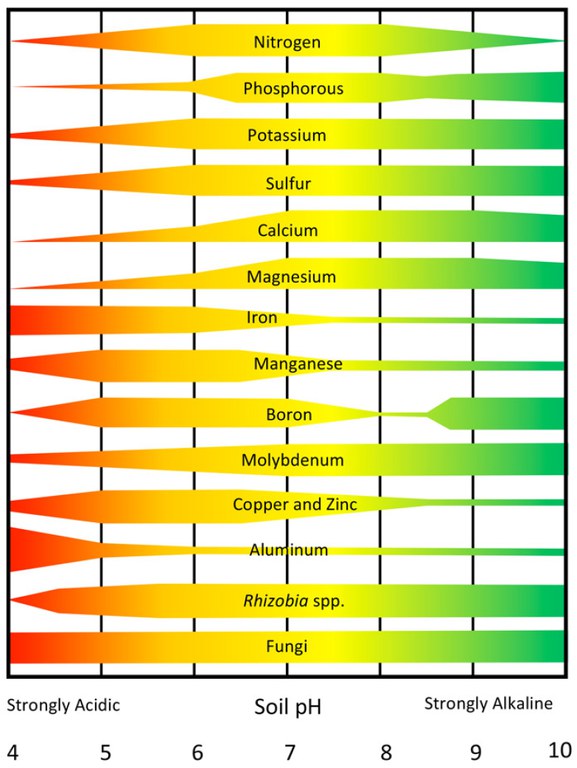
- Figure 2. The effects of soil pH on the availability of plant nutrients and selected groups of microorganisms. (Washington State University)
Aluminum Toxicity
Soil minerals are made up of Al and silica (Si) oxides (Al2O3, SiO2), which are combined into clay minerals (alumino-silicate minerals) in various proportions as the soil weathers. These minerals contain varying amounts of Al and Si.
Clay mineral structure can be variable, but simpler clay mineral structures such as kaolinite (1:1 Al:Si ratio) are less reactive than clay minerals such as montmorillonite (2:1 Si: Al ratio), which are more reactive. This reactivity is known as cation-exchange capacity (CEC), a measure of the soil’s negative charge, and is the ability of the soils or minerals to attract and hold positively charged basic cations such as Ca2+ and Mg2+.
Soils with a high CEC have a greater ability to hold basic cations and thus resist becoming more acidic. However, low CEC soils have less ability to resist acidification and become more acidic quickly. Soil organic matter also has a high CEC and helps buffer soils from becoming more acidic.
Figure 3 illustrates the formation of Al-species across a range of decreasing pH values. Hydrated aluminum species (combined with hydroxyl [OH-]) usually are not toxic to plants because their charge is too weak to displace basic cations (Ca2+, Mg2+) from soil exchange sites. As soil pH becomes lower, decreasing soil pH provides increasing H+ ion activity, which reacts with OH- ions combined with the Al3+ ion, stripping the OH- away from the Al3+, thereby increasing the charge on the Al-species to a +2 or +3 charge.
These species will displace Ca2+ and Mg2+ cation exchange sites and the Al3+ concentration increases in the plant root zone. Al3+ is not an essential plant nutrient, and as it increases in the soil solution, the concentrations of Ca2+ and Mg2+ decrease and plant growth is affected.
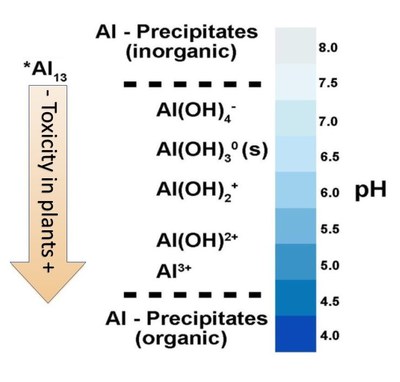
- Figure 3. The changes in Al3+ ionic species as soil pH changes. At pH less than 5, the unhydrated Al3+ is highly toxic to plants, but as soil pH increases, the hydrated Al (OH)x species have very low toxicity to plants. (Adapted from Bojorquez-Quintal, 2017. Modified by R. Alghamdi).
Aluminum Effects on Plants
High levels of Al3+ in the soil solution affect plant root development and growth. The main effects are an inhibition of root elongation by interfering with cell division at the root apex and lateral roots.
This results in a poor plant rooting system that interferes with its uptake, transport and use of nutrient elements such as Ca, Mg, P and K, as well as water. In addition, Al3+ bonds with P to form less available P, causing P deficiency symptoms. Therefore, plants exposed to toxic Al levels show poor growth, water stress and nutrient deficiencies.
A visual examination of the plant roots will show a poorly developed root system as well as poor root nodulation in legumes. Figure 4 shows soybean plants and their root system development under increasing aluminum toxicity conditions.

- Figure 4. Soybean plants and their root systems grown under field conditions where soil pH decreases (soil acidity increases) from pH 5.1 on the left to pH 4.5 on the right. (H. Weiser, Natural Resources Conservation Service).
Figure 5 illustrates the effects of the level of free Al3+ on aluminum-sensitive or -tolerant plant species. Most crop species are unaffected by free Al3+ down to pH 5, but below that pH, plants may be affected either directly by the Al3+ or by its interaction with availability of plant nutrients such as P resulting in decreased growth and productivity. Often this occurs without obvious visual plant symptoms.
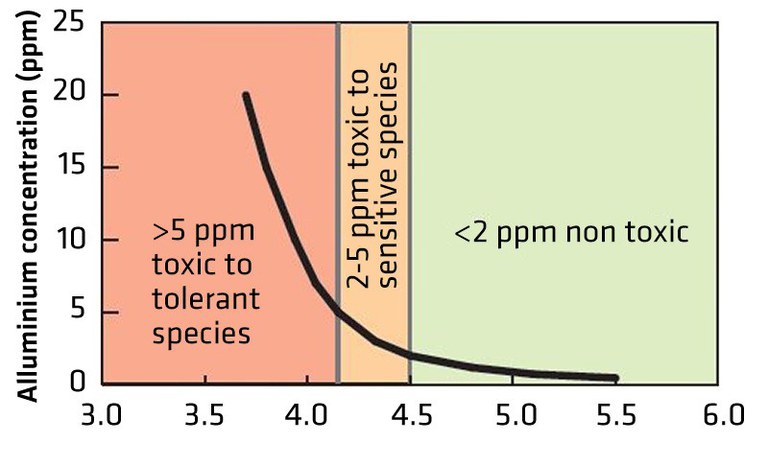
- Figure 5. The relationship between soil pH and free Al3+ in the soil and its toxicity to Al-sensitive and Al-tolerant plant species. (soilquality.org.au)
Identifying Acid Soils
Soil acidity can be determined by a routine soil pH test by a soil testing laboratory. These tests are available at the NDSU Soil Testing Laboratory and most commercial soil testing laboratories. Based on soil pH tests, the need for adding a lime amendment can be determined.
Additional Reading
Bojorquez-Quintal, E.B., C.E. Magaña, I.E. Machado and M.M. Estévez. 2017. Aluminum, a friend or foe of higher plants in acid soils. Front. Plant Sci. 8:1767. doi:10.3389/fpls.2017.01767. 18p.
Chien, S.H., R.L. Kallenbach and M.M. Gearhart. 2010. Liming requirement for nitrogen fertilizer-induced soil acidity: A new examination of AOAC Guidelines. Better Crops, 94(2):8-10.
Gazey, P. 2004? Soil acidity. https://soilquality.org.au. (Accessed Dec. 14, 2020).
Havilin, J.L., J.D. Beaton, S.L. Tindall and W.L. Nelson. 2005. Soil fertility and fertilizers, 7th ed. Pearson-Prentice Hall. p. 54.
Kissel, D.E., B.R. Bock and C.Z. Ogles. 2020. Thoughts on acidification of soils by nitrogen and sulfur fertilizers. Agrosyst. Geosci. Environ. 2020, 3:e20060. doi: 10.1002/agg2.2000.
Mamo, M., C. Wortmann and C. Shapiro. 2003. Lime use for soil acidity management. NebGuide G1504. Univ. of Nebr. Ext., Lincoln, Neb.
Neenu, S., and K.S. Karthika. 2019. Aluminum toxicity in soils and plants. Harit Dhara 2(1):15-19.
Wortman, C.S., M. Mamo and C.A. Shapiro. 2015. Management strategies to reduce
the rate of soil acidification. Univ. of Nebr. Ext. NebGuide G1503. Lincoln, Neb. June 2015. 4p.



