Sunflower Production Guide (A1995, Dec. 2020)
Foreword
The first edition of “Sunflower Production and Marketing Extension Bulletin 25” was published in 1975. Revised editions followed in 1978, 1985, 1994 and 2007. This publication replaces the publication titled “Sunflower Production,” which was published in 2007.
The purpose is to update information and provide a production and pest management guide for sunflower growers. This revised publication is directed primarily to the commercial production of sunflower, not to marketing and processing. It will attempt to give specific guidelines and recommendations on production practices and pest management based on current information.
This publication also is directed primarily toward sunflower production in the northern part of the Great Plains of the U.S. However, much of the information is relevant to other production areas.
All pesticides recommended have a U.S. Environmental Protection Agency label unless otherwise specified. This publication contains certain recommendations for pesticides that are labeled only for North Dakota. The users of any pesticide designated for a state label must have a copy of the state label in their possession at the time of application. State labels can be obtained from agricultural chemical dealers or distributors. Use pesticides only as labeled.
Acknowledgments
The editors are indebted to the contributors for writing sections of this publication. The editors also appreciate the efforts made by previous contributors because these previous sections often were the starting point for current sections.
This publication was compiled and published in cooperation with the National Sunflower Association (www.sunflowernsa.com).
Contributors
Patrick Beauzay, state IPM coordinator and research specialist, NDSU Extension, North Dakota State University, Fargo, ND 58105
Gary Brewer, former department chair and professor, Department of Entomology, North Dakota State University, Fargo, ND 58105
Ryan Buetow, Extension cropping systems specialist, NDSU Research Extension Center, Dickinson, ND 58601
Anitha Chirumamilla, Extension agent, agriculture and natural resources, Cavalier County, Langdon, ND 58249
Greg Endres, Extension cropping systems specialist, NDSU Research Extension Center, Carrington, ND 58421
Dave Franzen, Extension soils specialist, NDSU Extension, North Dakota State University, Fargo, ND 58105
Bob Harveson, Extension plant pathologist, University of Nebraska, Scottsbluff, NE 69361
Kenneth Hellevang, Extension agricultural engineer, NDSU Extension, North Dakota State University, Fargo, ND 58105
Karl Hoppe, Extension livestock systems specialist, NDSU Research Extension Center, Carrington, ND 58421
Brent Hulke, sunflower breeder, U.S. Department of Agriculture – Agricultural Research Service (USDA-ARS), North Dakota State University, Fargo, ND 58105
Joe Ikley, Extension weed specialist, NDSU Extension, North Dakota State University, Fargo, ND 58105
Hans Kandel, Extension agronomist, NDSU Extension, North Dakota State University, Fargo, ND 58105
Page Klug, research wildlife biologist, USDA-Animal and Plant Health Inspection Service-Wildlife Service National Wildlife Research Center, North Dakota Field Station, North Dakota State University, Fargo, ND 58105
Jan Knodel, Extension entomologist, NDSU Extension, North Dakota State University, Fargo, ND 58105
Sam Markell, Extension plant pathologist, NDSU Extension, North Dakota State University, Fargo, ND 58105
Febina Mathew, oilseeds plant pathologist, South Dakota State University, Brookings, SD 57007
John Nowatzki, ag machine systems specialist, NDSU Extension, North Dakota State University, Fargo, ND 58105
Frayne Olson, Extension crops economist, NDSU Extension, North Dakota State University, Fargo, ND 58105
John Sandbakken, Executive director, National Sunflower Association, Mandan, ND 58554
Tom Scherer, Extension agricultural engineer, NDSU Extension, North Dakota State University, Fargo, ND 58105
Former editors: David W. Cobia, David E. Zimmer, Marcia McMullen and Duane R. Berglund
Former contributors:
Ron R. Allen, Roger Ashley, William S. Ball, James Bauder, Duane R. Berglund, Al Black, Carl Bradley, Lawrence Charlet, David W. Cobia, William Danke, Alan Dexter, Carl Fanning, Gerhardt N. Fick, George Flaskerud, Basil Furgala, Phil Glogoza, Thomas Gulya, James Hanzel, James Helm, Harvey J. Hirning, Edna T. Holm, Vernon L. Hofman, David H. Kinard, Larry Kleingartner, Arthur Lamey, Greg Lardy, George Linz,
Darnell Lundstrom, Dean McBride, Hugh McDonald, Jerry Miller, John Nalewaja, Berlin Nelson, David M. Noetzel, William K. Pfeifer, Lyle Prunty, Charlie E. Rogers, LeRoy W. Schaffner, Albert Schneiter, Robert and Jay Schuler, John T. Schulz, Don Tanaka, Tommy E. Thompson, Sebastian Vogel, Howard D. Wilkins, David E. Zimmer, Richard Zollinger and Joseph C. Zubriski.
Introduction
Hans Kandel
Three primary types of sunflower are grown: (1) oilseed for vegetable oil production, (2) nonoilseed for human food and bird food markets and (3) Conoil, which can be used for the dehull confection, oil or bird food markets. The oilseed hybrids may be of three fatty acid types: linoleic, mid-oleic (NuSun) or high oleic. They are usually black seeded and have a thin hull that adheres to the kernel.
Seed of the oilseed varieties contains from 38% to 50% oil and about 20% protein. Some black-seeded oil types go into the hulling market for birdseed. Nonoilseed sunflowers also has been referred to as confectionery sunflower, and seeds usually are white striped and large.
Nonoilseed sunflowers generally have a relatively thick hull that remains loosely attached to the kernel, permitting more complete dehulling. Seed of the nonoilseed hybrids generally is larger than that of the oilseed types and has a lower oil percentage and test weight. Sunflowers are a major source of vegetable oil in the world.
Historical Perspective
Sunflower, native to North America, grows wild in many areas of the U.S. Sunflower has a long and varied history as an economic plant, but the time and place of its first cultivation is uncertain. Sunflower was used by North American inhabitants before colonization of the New World. Spanish explorers collected sunflowers in North America, and by 1580, it was a common garden flower in Spain. Early English and French explorers, finding sunflower in common use by the native Americans, introduced it to their respective lands. It spread along the trade routes to Italy, Egypt, Afghanistan, India, China and Russia.
Sunflower developed as a premier oilseed crop in Russia and has found wide acceptance throughout Europe. Oilseed sunflower has been an economically important crop in the U.S. since 1966. Before 1966, sunflower acreage in the U.S. was devoted primarily to nonoilseed varieties.
The center of sunflowers’ origin has been identified as being limited to the western Plains of North America, but whether the domesticated type originated in the Southwest or in the Mississippi or Missouri River valleys has not been determined. The wild form of the cultivated sunflower is well-known, which is not true with most of our cultivated crop species today.
The Native Americans used sunflower as a food source before the cultivation of corn. Sunflower also was used as a medicinal crop, source of dye, oil for ceremonial body painting and pottery, and as a hunting calendar. When sunflower were tall and in bloom, the bison fed on it, and according to stories told, the fat and the meat were good.
Cultivation of sunflower was undertaken by New World settlers as a supplementary food. Later, sunflowers were grown primarily as a garden ornament. They also were grown as an ensilage crop in the late 1800s and early 1900s.
Expanded world production of sunflower resulted primarily from development of high-oil varieties by plant scientists and the development of hybrids. Sunflower is widely grown in the world where the climates are favorable and a high-quality oil is desired.
Taxonomy
The cultivated sunflower (Helianthus annuus L.) is one of the 67 species in the genus Helianthus. All are native to the Americas and most are found in the U.S. It is a member of the Asteraceae family and has a typical composite flower (Figure 1).
The basic chromosome number for the Helianthus genus is 17. Diploid, tetraploid and hexaploid species are known.
The majority of the species are perennial, with only about a dozen annual species. Plant breeders have made interspecific crosses within the genus and have transferred such useful characteristics as higher oil percentage, cytoplasmic male sterility for use in production of hybrids, and disease and insect resistance to commercial sunflower.

Growth Stages
The division of growth into vegetative and reproductive stages as developed by Schneiter and Miller is shown in Figure 2. This scheme is important because it gives producers, scientists and the industry a common basis to discuss plant development.

- Figure 2. Stages ofsunflower development.(A.A. Schneiter and J.F. Miller)

Description of Sunflower Growth Stages
The total time required for development of a sunflower plant and the time between the various stages of development depends on the genetic background of the plant and growing season environment (Table 1). When determining the growth stage of a sunflower field, the average development of a large number of plants should be considered (Table 2).
This staging method also can be used for individual plants. In stages R7 through R9, use healthy, disease-free heads to determine plant development if possible because some diseases can cause head discoloration. Also, in a number of hybrids, the stay-green characteristic is present, which means the yellowing or browning of the bracts may not be a good indicator of plant maturity.
Table 1. Growing degree days: sunflower growth and development.
Table 2. Description of sunflower growth stages.
Production
World Production
John Sandbakken and Hans Kandel
Sunflower is native to North America but commercialization of the plant took place in Russia. Sunflower oil is the preferred oil in most of Europe, Mexico and several South American countries.
Major producing countries or areas are Ukraine, Russia, European Union, Argentina, Turkey and the U.S. (Figure 3). These countries/areas of the world produced in 2019 about 86% of the world’s oilseed and nonoilseed sunflower.
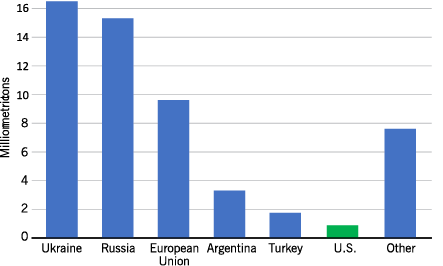
- Figure 3. 2019 world production of all sunflowers.

U.S. Production
Acreage
The first sustained commercial production of oilseed sunflower in the U.S. occurred in 1966, when about 6,000 acres were grown. Total combined acreage of oilseed and nonoilseed sunflower increased gradually in the late 1960s and expanded rapidly in the 1970s, reaching a peak in 1979 at 5.5 million acres. The U.S. share of world production has declined as production in Argentina and other countries has increased.
The bulk of U.S. sunflower production occurs in North Dakota and South Dakota. Other contributing states include Kansas, Minnesota, Colorado, Texas and Nebraska (Table 3). The majority of the acreage harvested is for oil production versus nonoil uses (Table 4).
Table 3. Total planted sunflower acreage by states 1995-2019.
Seed Yield Per Acre
Annual average North Dakota sunflower yields from 1990 to 2019 ranged from 840 to 1,750 pounds per acre for oilseed and from 780 to 1,860 pounds per acre for nonoilseed sunflower. Average yield for all oil and nonoil sunflower per acre for the 1990-2019 period was 1,383 pounds per acre. Yearly variability is shown in Figure 4.
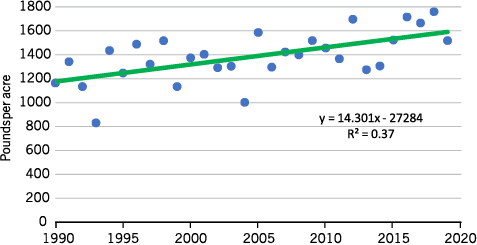
- Figure 4. Average North Dakota sunflower yield 1990-2019 in pounds per acre.

Prices
Historically, sunflower depended heavily on the export market for either seed or oil. With the advent of NuSun and high oleic sunflower, the market has switched almost exclusively to a U.S. and Canadian market. Both of these oils are very stable and do not require hydrogenation as do competitive oils, such as traditional soybean and canola oils, when used in a frying application. Sunflower prices now are more determined by their relationship to corn oil prices.
Large domestic users tend to buy in advance. Thus prices are not directly affected by the Chicago soybean oil contract and are not as likely to be as volatile. More opportunities are available to presell a portion of the crop well before planting begins. This ensures a domestic user of a supply and allows a producer to “lock in” a price for a portion of his production.
Storage of sunflower is necessary. The domestic market needs a 12-month supply of oil and crushers will need a steady supply of seed. Crushers likely will have to provide producers with storage premiums for delivery in the out-of-harvest months.
Oilseed sunflower producers have the advantage of multiple market options: the hulling market, the crush market or the bird food market. Supply and demand drive prices in all three markets.
Nonoilseed sunflower production is geared to the “in-shell” markets. Nonoilseed hybrids produce a significant level of large seeds. Growers often are paid on a percentage of large seed. Quality standards for confection sunflower are high and allow little tolerance for off-color and insect damage.
Sunflower Marketing Strategy
Frayne Olson
Sunflower marketing strategies usually use the cash forward contract for locking in a price prior to harvest. Use of this contract may be appropriate on a portion of the sunflower crop, but on-farm storage for sale at a later date is also common. Storage costs and the risk of quality loss must be weighed against the potential for higher prices in the future.
Production Contracts
Production contracts are common in the sunflower industry. This section provides a brief overview of key contract provisions that should be reviewed and understood before entering into a production contract.
Understand what you are signing – Reading and understanding contract provisions always is important because they describe the rights and responsibilities of both parties in the agreement. Considerable differences can occur in contract terms among companies and contract provisions often change through time. Discussing contract provisions with the buyer before signing a contract can prevent misunderstandings and help maintain a strong working relationship.
Production requirements – Most sunflower production contracts specifically require the farmer (seller) to use accepted agronomic production practices and apply only registered crop protection products. Some contracts also include a list of acceptable hybrids or require the seed to be purchased from the company (buyer). Because confection sunflowers are used as human food, the expectation is that food safety standards and testing will become more stringent in the future. Stricter food safety requirements likely will lead to contracts including more detailed production provisions.
Act-of-God clause – Some sunflower contracts contain an act-of-God clause, which releases the farmer (seller) from the terms of the contract due to an act of God, such as hail, drought, flood or disease. An act-of-God clause normally only covers the production shortfall below the contracted amount. The farmer (seller) still is expected to deliver the available production from the contracted acres.
The farmer (seller) must notify the company (buyer) as soon as possible when a potential production problem occurs to ensure that this contract provision is enacted. Many contracts require the farmer to provide written notice within 10 days of an event.
Grading and quality standards – The U.S. Department of Agriculture’s Federal Grain Inspection Service standards are the core standards used to trade sunflower. However, some domestic and international end users are beginning to request more detailed grading and quality specifications. Grading and quality specifications should be listed clearly in the contract. If they are not, be sure to ask the buyer for a copy of the grading and quality standards that will be used.
Delivery period – Production contracts typically require delivery at harvest during a pre-specified delivery period or on a “buyer’s-call” basis. Harvest delivery refers to delivery directly from the field to the agreed-upon delivery point during the normal harvest period. This is the most common type of contract in the sunflower industry. Buyers in other crop sectors commonly offer alternative prices for pre-specified delivery windows, such as the first half of November, to better match deliveries with expected shipments. Buyer’s-call refers to an open-ended delivery schedule in which the company (buyer) will determine the delivery period and schedule deliveries with the farmer (seller) when needed. Buyer’s-call typically requires the farmer to store the contracted production until delivery is requested.
Pricing and payment – Sunflower production contracts typically use a fixed-base price for the contracted production. Price premiums or discounts can be used to adjust for grade and quality differences. Some specific premium or discount rates are not known until the time of delivery. Payment generally is made a short time after all of the contracted production has been delivered. However, delayed payment or deferred payment options often are available.
Hybrid Selection and Production Practices
Hybrid Selection
Brent Hulke and Hans Kandel
Selection of sunflower hybrids (Figure 5) to plant is one of the most important decisions a producer must make each season. In addition to intended market, variables such as yield, maturity, dry down, standability, herbicide tolerance, and pest and disease resistance, should be considered.

- Figure 5. A hybrid seed production field of sunflower. Female and male parents are planted in alternate strips across the field. (Marcia McMullen, NDSU)
.jpg)
Sunflower Market Types
Several different market classes of hybrids exist. Oilseed hybrids currently marketed in North America are either “NuSun” (mid-oleic) or “high oleic.” NuSun sunflower hybrids will produce an oil with more than 55% oleic fatty acid, a monounsaturated omega-9 fatty acid and 15% to 35% linoleic fatty acid. High oleic sunflower has a high proportion of oleic acid in the oil, typically above 85%, and the oil has a long shelf life.
Nonoilseed hybrids also are available for in-shell markets, and most have a high linoleic (traditional) oil profile. Confection hybrids are characterized by having large seed, with a distinctive color striping on the hull. Hybrids with very long, large seed are in demand for the export market.
Producers must be careful to set their combine concave widths properly to avoid hull damage on these hybrids. Producers generally plant nonoilseed hybrids at a lower plant population and increase insect scouting and control to maintain high kernel quality. Contracts are available to producers interested in planting nonoilseed hybrids.
Conoil hybrids, which are a cross between oilseed and confection type, also are available for producing dehulled kernel products and “SunButter” under contract.
Criteria for Hybrid Selection
Growers should use several criteria in hybrid selection. Seed yield potential is an important trait to consider when looking at an available hybrid list. Yield trial results from university experiment stations, National Sunflower Association-sponsored trials and commercial companies should identify consistently high yielding hybrids for a particular area. For North Dakota, information is available on the variety trial web site www.ag.ndsu.edu/varietytrials/sunflower.
Oil percentage should be another trait to consider in oilseed hybrid selection. Several environmental factors influence oil percentage, but the hybrid’s genetic potential for oil percentage in your region is one of the most important considerations for hybrid choice because of the oil premium/discount structure.
Maturity and dry down should be considered when deciding what hybrid to plant. Maturity is especially important if planting is delayed, being mindful of the average killing frost in your area. Yield, oil content and test weight often are reduced when a hybrid is damaged by frost before it is fully mature.
An earlier hybrid likely will be drier at harvest than a later hybrid, thus reducing drying costs and potentially allowing for earlier harvest to avoid blackbird feeding on the sunflowers. Also, consider planting hybrids with different maturity dates as a production hedge to spread risk and workload at harvest.
The most economical and effective means to control sunflower diseases and other pests is planting resistant or tolerant hybrids and considering a minimum of three to four years’ rotation between successive sunflower crops. Hybrids are available with resistance to rust, Verticillium wilt and certain races of downy mildew.
Whole-plant stability traits, such as root strength and stalk health, are important to reduce lodging, stem diseases and infestation from insects such as the Dectes stem borer. Hybrid selection may include selecting a hybrid with resistance to certain postemergence herbicides: Express or Beyond. This nontransgenic resistance was derived from the wild species of sunflower or from mutagenesis.
Production Practices
Seed Quality
Hans Kandel
High quality, uniform seed with high-germination, known hybrid varietal purity and freedom from weed seeds and disease should be selected to reduce production risks. The standard germination test provides an indication of performance under ideal conditions but is limited in its ability to estimate what will happen under stress.
Accelerated aging is another method used to evaluate seed vigor. Any old or carry-over seed should have both types of tests conducted.
Seed size designations are fairly uniform across companies. Most seed is treated with a fungicide and insecticide to protect the germinating seedling. Seed should be uniformly sized to allow precision in the planting operation.
Soils
Sunflower is adapted to a variety of soil conditions but grows best on well-drained, high water-holding capacity soils with a nearly neutral pH (pH 6.5-7.5). If pH is below 5.5, we recommend you apply lime.
Soil Fertility
David Franzen
The following recommendations are extensive revisions of any guidelines provided in North Dakota before 2016. North Dakota sunflower nitrogen (N) recommendations are based on the results from 52 experiments: four North Dakota N rate experiments in 2012-13, 40 North Dakota N and phosphorus (P) rate experiments in 2014-15, and eight N and P rate experiments in South Dakota in 2014-15, with 48 total experiments taken to yield.
The results from these studies indicate that yield is independent of N rate, meaning that any formula of (Yield X (a factor) = N rate) is incorrect and nonpredictive. In addition, the new recommendations account for the greater susceptibility to lodging with higher N rates and the relationship between higher N rates and increased sunflower disease.
Although in the absence of high wind and disease, higher yield might be possible with higher N rates than those in the recommendation tables or in the sunflower N calculator www.ndsu.edu/pubweb/soils/sunflower. Sunflower growers assume a greater lodging and disease risk if they decide to use higher rates.
Nitrogen
The new sunflower recommendations are regionally and tillage-based. Because all soil fertility is local, sunflower growers in states other than North Dakota need to review their own locally generated, date-driven recommendations for their farms.
Perhaps due to the deeper rooting nature of sunflower, soil texture had less effect on yield, compared with clay texture effect on corn N use efficiency in previous studies. However, at most sites, which were planted to sunflower for the first time in more than 30 years or did not have a deep-rooted crop such as sugarbeet or sunflower within the past five years, yield response was very slight, if any increase occurred. Oil content tended to be near or below 40%, even with zero added N, indicating significant deep soil N was present in these fields.
The western North Dakota site that was not planted to sunflower for at least five years experienced a major drought in 2012, followed by relatively wet seasons in 2013-2015, which may have moved N downward in the soil. In the sunflower crop of 2015, the deeper N in this field likely was tapped, resulting in no yield increase with higher N rates and relatively low oil content in the seed. The sites in eastern North Dakota without sugarbeet or sunflower in recent memory likewise did not have increased yield with higher N rates and had oil content that, even at the zero N rate, was below 40%.
If practical, new fields of sunflower should be sampled to at least a 4-foot depth to see if deeper N should be considered in N fertility recommendations. About 30 pounds of N from 2 to 4 feet in depth should be assumed but not subtracted from N recommendations. But if the 2- to 4-foot soil nitrate test is greater than 30 pounds N per acre, then the extra N should be subtracted from the total N recommendation.
If deeper soil sampling is not practical, an N nonlimiting area of a full rate of N based on the N calculator or the N recommendation tables should be applied, and about half the N calculator rate should be applied to the rest of the field. Using an active-optical sensor (SPAD meter) or “best-guess” assessment of color differences and vigor of the N nonlimiting area, compared with the rest of the field, an N application could be made or not made from about stage V8 to R1 (approximately V12 to V14) using a side-dress applicator or high-clearance applicator (Figure 6).
In fields without sunflower, sugarbeet or another deep-rooted crop such as safflower, no additional N likely needs to be applied. Soil sampling to 2 feet in depth is very important for N recommendations in North Dakota.
.jpg)
Figure 7 shows yields with N rate and soil test nitrate-N to the 2 feet in depth considered for N fertility recommendations. The N recommendations are based on differences in the response of sunflowers in eastern North Dakota compared with those in western North Dakota (Figure 8, map of North Dakota).
Although no sunflower studies were conducted in the Langdon area, previous research in wheat indicates that these soils contain small pieces of shale that release N during the growing season, and N rates should be reduced in this area for sunflower production below eastern North Dakota rates.
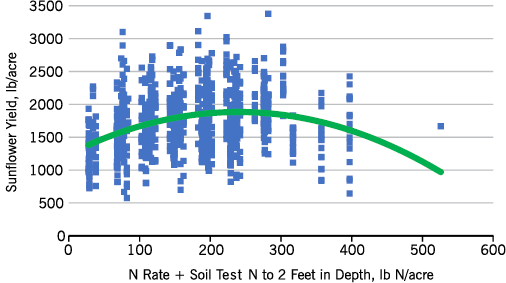
- Figure 7. Sunflower N rate trial results, 2014, with soil test nitrate-N to 2 feet in depth considered.

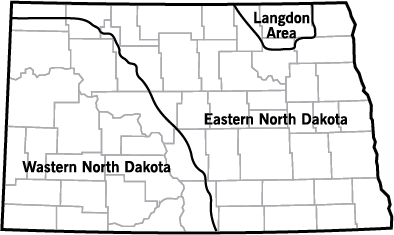
- Figure 8. Approximate areas described by eastern North Dakota and western North Dakota sunflower recommendations.

Eastern no-till sunflower N responses are similar to those of conventional till (Figures 9 and 10), although the N required to reach maximum yield is less. Both no-till and conventional till yield responses are quadratic, which means that after reaching maximum yield, yields decline with added N. This decline could be due to lodging or increased susceptibility to disease. The yields were determined on hand-harvested plots, and heads of all lodged plants were recovered.
Western no-till sunflower response is different than those in the east (Figure 11), with positive yield responses even at what is currently considered excessive N rates. However, N recommendations also consider the hazard of greater lodging and perhaps greater disease severity with high N rates. In a commercial field, some harvest loss is likely with lodging, so the upper limits of the N rate were reduced in the recommendation tables to limit lodging loss.
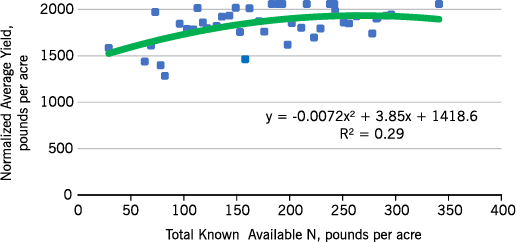
- Figure 9. Eastern North Dakota oil-seed sunflower normalized yield within site response to total known available N rate, long-term no-till sites, 2012-2015.
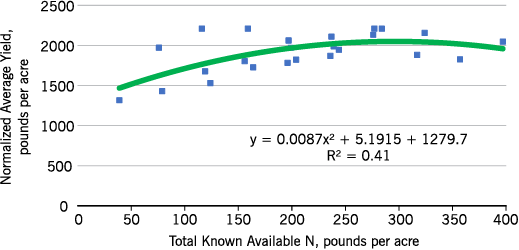
- Figure 10. Eastern North Dakota nonoilseed sunflower normalized yield within site response to total known available N, conventional tillage, 2012-2015.

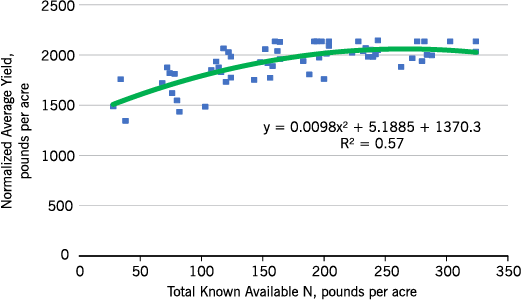
- Figure 11. Western North Dakota oilseed sunflower normalized yield within site, response to total known available N, long-term no-till, 2014-2015.


Although generally white mold and downy mildew seemed to be related to the N rate, only one site was scored for disease. This site, north of Dickinson, N.D., was rated for sunflower rust, caused by the fungus Puccinia helianthi, at harvest. An application of 80 pounds per acre of N resulted in significantly more rust on the leaves compared with the control of zero N application.
Excessive wind resulting in sunflower root lodging or stalk breakage does not happen in every sunflower field every year, but lodging is a concern of most sunflower growers. Several sites were affected by wind each year in our studies, and lodging severity was directly related to the N rate (Figure 12).
Although in our N rate experiments, all heads were included in yield, sunflower growers would suffer a decrease in harvest efficiency when sunflowers are on or near the ground. Therefore, the N rate is capped in the no-till N recommendations, even though small increases in yield and marginal profit might be possible with greater N rates in nonwind-affected growing seasons.
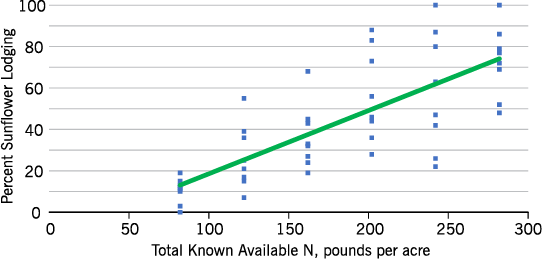
- Figure 12. Percent of sunflower lodging with increased available N at Bottineau, 2015.

Seed oil content of oilseed sunflowers always decreased with the N rate, regardless of yield response. The economic impact of available N on yield response, seed oil response and N cost is factored into each N recommendation. Highest N rates are moderated due to possible harvestable yield reduction due to lodging. The N rate recommendations are available in Tables 5-7.
Table 5. Eastern long-term no-till oilseed sunflower N recommendations based on N cost and sunflower price. For the nonoilseed (confection) sunflower N rate, add 10 pounds of N per acre to these values, except zero values.
The database that was accumulated to produce these recommendations did not include any conventional tillage sites in the western region. Best recommendations for conventional tillage likely would be N rates similar to the no-till rates, but growers would need to expect lower yield, compared with their no-till neighbors in drier seasons.
The Langdon region was not represented in our database, but previous N rate experiments with wheat indicated about a 50 pound per acre N contribution from the slow-release mineralizable N within the shale pieces abundant in soils of this region. Therefore, for the Langdon region, subtract 50 pounds from the rates of eastern no-tillage oil sunflower as indicated in Table 5 or eastern conventional tillage nonoilseed sunflower in Table 7.
Table 6. Western long-term no-till oilseed sunflower N recommendations based on N cost and sunflower price. For the nonoilseed (confection) sunflower rate, add 10 pounds of N per acre to these values, except zero values.
Table 7. Eastern conventional-till oilseed sunflower N recommendations based on N cost and sunflower price. For the nonoilseed confection sunflower N rate, add 10 pounds of N per acre to these values, except zero values.
Phosphorus
High soil phosphorus (P) levels and high rates of P fertilizer have not been found necessary for high sunflower yields in U.S. studies. Fallow does not appear to induce P deficiency in sunflower as it does in corn, despite the high reliance of sunflower on mycorrhiza.
Forty site-years of N and P rate experiments were conducted in western Nebraska during 1993 and 1994. Researchers found no effect of the P rate on sunflower yield at any location. Most of the Nebraska sites had medium to high soil P levels, but some sites were classified in the low soil P range and yet did not respond to P.
Of the 48 P rate experiments in North Dakota and South Dakota, only three had statistically greater yield, with P rates from 60 to 90 pounds of P2O5 per acre, compared with the check. At only one site was the yield increase economically beneficial when the cost of the P fertilizer was considered.
The recommendation for P fertilizer for sunflower for North Dakota therefore is zero. Although the application of P fertilizer can be made and does not decrease yield, neither does it economically increase yield.
Soil test P was not related to P response. Sunflower P removal in grain is very low, with only about 10 pounds of P2O5 per acre removed with a 2,000-pound-per-acre seed yield.
Potassium
Potassium (K) has been studied little for sunflower in the northern sunflower growing region. Around the world, K rate studies indicate that about 150 parts per million (ppm) from the standard K test in northern sunflower growing region is sufficient for maximum sunflower yield.
South Dakota data from corn K rate studies indicate that if soil K levels are below 150 ppm, about 100 pounds per acre of 0-0-60 is necessary to maximize yields in any year. Therefore, if the soil test is below 150 ppm, the K fertilizer rate is a flat 100 pounds per acre rate of 0-0-60, or 60 pound per acre of K2O.
Sulfur
Sulfur (S) has become a common nutrient that is deficient for many crops in North Dakota. Although sunflower is deeply rooted, and in many soils, the groundwater is high in sulfates, sunflower may be susceptible to early season deficiency.
The S soil test is not diagnostic. A better prediction for possible S deficiency would be noting fall rainfall, winter snowfall, spring snowmelt and rainfall before planting. If any high precipitation is experienced, the possibility of S deficiency is greater.
Loam and coarser-textured soils are most susceptible to S deficiency. If S is anticipated to be deficient, an application of 10 pounds of S as a sulfate or thiosulfate source is recommended as preplant or postemergence before the reproductive stage (R1) of growth.
Elemental S application is not recommended. Do not apply any thiosulfate fertilizer with the seed. Also, sulfur is a spring fertilizer, so fall application is not recommended due to the likelihood of leaching in the spring before planting.
Other Nutrients
Of all the micronutrients, sunflower is most susceptible to boron (B) deficiency, from reports around the world. However, in North Dakota, B rate experiments with soil B levels as low as 0.2 ppm showed no sunflower yield response with B application. Thus, no micronutrient application, including B application, is needed in North Dakota.
Organic Sunflower Nutrient Management
With the current market for organic foods of all kinds, sunflower lends itself well to certified organic production. For the requirements in North Dakota, contact the North Dakota Organic Advisory Board for a list of U.S. Department of Agriculture-vetted certifiers (www.nd.gov/ndda/marketing-information-division/organics).
Organic systems usually are depleted of N, but soil sampling can be helpful in determining the need for supplemental N amendments. One strategy organic growers use is to grow an early maturing crop, such as barley or winter wheat, the year before sunflowers and grow a cover crop of a fast-growing annual legume directly after grain harvest.
Compost also can be used to great advantage. The compost should come from a reliable source, where the manure was brought to temperatures that kill weed seed. Well-made composts can be sampled and analyzed for N content so that a better estimate can be made of N release during the sunflower growing year. A guide to producing and managing compost is available at www.ag.ndsu.edu/manure/documents/nm1478.pdf.
Compost also will contain substantial potassium, which might be limiting on very sandy soils, as well as sulfur. Sulfur fertilizers that also can be used include gypsum from natural sources and potassium sulfate (check with the North Dakota Organic Advisory Board for details).
Water Requirements for Sunflower
Hans Kandel
Sunflower plants have deep roots and extracts water from depths not reached by most other crops; thus, sunflower is perceived to be a drought-tolerant crop. Sunflower has an effective root depth of around 4 feet but can remove water from below this depth.
Research on side-by-side plots has shown that sunflower is capable of extracting more water than corn from an equal root zone volume. With its deep root system, it also can use N and other nutrients that leach below shallow-root crops; thus, sunflower is a good crop to have in a rotation.
Seasonal water use by sunflower averages about 19 inches under irrigated conditions. Under dryland conditions, sunflower will use whatever stored soil moisture and rain that it receives during the growing season. When access to water is not limited, small grains use 2 to 3 inches less total water than sunflower during the growing season, whereas soybean water use is slightly greater. Corn uses 1 to 4 inches, and sugarbeet use 2 to 6 inches more than sunflower during the growing season.
These total water use values are typical for nondrought conditions in southeastern North Dakota. Small grains use the least total water because they have the fewest number of days from emergence to maturity. Sunflower and soybean have an intermediate number of days of active growth and corresponding relative water use. Corn ranks above sunflower in growth days and water use, while sugarbeet rank highest in both categories.
However, water use efficiency does vary among these crops. Comparative water use efficiency measured as grain (pounds per acre or lb/A) per inch of water used on three dryland sites and two years in eastern North Dakota was 119, 222, 307, 41, 218, 138 and 127 for sunflower, barley, grain corn, flax, pinto bean, soybean and wheat, respectively. These results indicated that corn had the highest water use efficiency, sunflower and wheat were intermediate and flax the lowest.
Fertility has little influence on total water use, but as fertility increases, water use efficiency increases because yield increases. Yield performance has been shown to be a good indicator of water use efficiency of sunflower hybrids; higher yielding hybrids exhibit the highest water use efficiency.
Soil Water Management for Dryland Sunflower
Hans Kandel
Management practices that promote infiltration of water in the soil and limit evaporation from the soil generally will be beneficial for sunflower production in terms of available soil moisture. Leaving stubble during the winter to catch snow and minimum tillage are examples.
Good weed control also conserves moisture for the crop. The use of post-applied and pre-emergence herbicides with no soil incorporation also conserves moisture when growing sunflower.
Sunflower has the ability to exploit a large rooting volume for soil water. Fields for sunflower production should be selected from those with the greater water-holding capacity and soils without layers that may restrict roots.
Water-holding capacity depends mainly on soil texture and soil depth. The loam, silt loam, clay loam and silty clay loam textures have the highest water-holding capacities. Water-holding capacity of the soils in any field can be obtained from county soil survey information available from local U.S. Department of Agriculture’s (USDA) Natural Resources Conservation Service (NRCS) offices or online by Googling NRCS web soil survey.
Sampling or probing for available soil moisture before planting also can help select fields for sunflower production. With other factors being equal, fields with the most stored soil moisture will have potential for higher yields. Where surface runoff can be reduced or snow entrapment increased by tillage or residue management, increases in stored soil moisture should occur and be beneficial to a deep-rooted crop such as sunflower.
Irrigation Management
Tom Scherer
Irrigation of sunflower is not common but sunflower will respond to irrigation, especially during hot, dry years. About 1,500 acres of sunflowers are irrigated in North Dakota each year.
Long-term yield data from the Carrington Research Extension Center for irrigated and dryland oil-type hybrids show an average yield differential of about 500 pounds per acre. However, in some years, the irrigated trials yielded 1,500 pounds per acre more than the dryland plots, and in some years, the dryland plots actually yielded more than the irrigated plots. Irrigation increases the risk of disease, primarily white mold (Sclerotinia).
Annual water use by sunflowers averages about 19 inches, which is provided by a combination of stored soil water, effective rain and applied irrigation. Average daily water use will increase from about 0.03 inch soon after emergence to more than 0.27 inch from head emergence to full seed head development. However, during July and August, water use on a hot, windy day can exceed 0.32 inch.
Research has shown that sunflower yield is most sensitive to moisture stress during the flowering period (R2 to R5.9 reproductive stages) and least sensitive during the vegetative period (emergence to early bud). Research has shown that excessive soil water depletion during the R2 to R5.9 period resulted in a 50% yield reduction.
If soil water content is near field capacity at planting, research indicates that the first irrigation can be delayed until the root zone soil moisture is about 70% depleted. However, if pumping capacity is low (less than 6 gallons per minute per acre for a center pivot), a lesser depletion is advisable due to inadequate “catch-up capacity.”
Irrigations during the critical bud to ray-petal appearance (R2 to R5.0) period should be scheduled to maintain a low soil moisture stress condition (40% to 50% depletion). If possible, less frequent with higher application amounts should be used from R5.1 to R5.9 due to susceptibility to head rot from Sclerotinia (white mold). After R5.9, soil moisture depletion can approach 70% with little or no depression in yield.
Research indicates that the seed yield versus crop water use (ET) exhibits a linear relationship with a slope averaging 190 pounds per acre-inch. This means that when irrigation is required (during dry periods), every additional inch of water will increase seed yield by about 190 pounds per acre. A yield increase of 50% or more with irrigation may be expected almost every year on sand — loam to loamy sand soils.
However, a seed yield increase from irrigation may not always occur on soils with higher water-holding capacities and with adequate precipitation. Adequate soil fertility is very important in achieving the higher yield potential under irrigation.
Management of applied irrigation water requires the combination of periodic soil moisture measurement with a method of irrigation scheduling. Soil moisture can be measured or estimated in a variety of ways. The simplest is the traditional “soil feel” method that is an art developed through time with extensive use and experience. However, now many commercial soil-water measuring devices are available and can be accessed remotely via a smartphone or tablet.
Irrigation scheduling using the checkbook method is common. The checkbook method provides a continuous account of the water stored in the soil. Soil water losses due to crop use and soil surface evaporation are estimated each day based on the maximum temperature and the days since crop emergence. Precipitation and irrigation are measured and added to the soil water account each day. North Dakota Extension publication AE792 provides detailed instructions of the checkbook method. It is online at www.ag.ndsu.edu/publications/crops/irrigation-scheduling-by-the-checkbook-method-1
In addition to the printed version, two electronic versions have been developed. One is a spreadsheet version and the other is a site-specific version available on the North Dakota Agricultural Weather Network (NDAWN) website (https://ndawn.ndsu.nodak.edu/). Look under “Applications” in the left-hand menu. More details about the electronic versions can be found at www.ag.ndsu.edu/irrigation/irrigation-scheduling.
Another form of irrigation scheduling is to use estimated daily water use values for sunflower (Table 8). For example, during the eighth week after emergence, if the daily air temperature were 85 F on a particular day, sunflower water use for that day would be 0.25 inch.
This method, sometimes called the “water use replacement method,” is based on obtaining daily estimates of sunflower water use and accurately measuring the amount of rain received on the field. When a certain amount of water has been used by the crop, irrigation is initiated. More accurate local sunflower water use values are available on the NDAWN website. Look under “Applications” in the left side menu.
Table 8. Average daily water use for sunflower in inches per day based on maximum daily air temperature and weeks past emergence.
Tillage, Seedbed Preparation and Planting
Greg Endres, Hans Kandel and Ryan Buetow
Tillage and Seedbed Preparation
Sunflower, like other crops, requires proper seedbed conditions for optimum plant establishment. Seedbed preparation, soil tilth, planting date, planting depth, row width, seed distribution and plant population should be nearly correct as conditions permit. If an improper plant stand exists, this likely will cause production challenges throughout the growing season.
Maintaining a seedbed with consistent soil properties, especially adequate moisture, is important if producers expect a field to have uniform seed germination and plant emergence. Soil temperature near 50 F is required for seed germination. Poor germination and emergence will influence the need for and the effectiveness of future management practices.
Excessive tillage should be avoided where tillage is used to prepare the seedbed or to incorporate preplant herbicides. Excessive tillage will break down soil structure, cause compaction and crusting problems, reduce aeration, restrict water movement and provide conditions favorable for infection by downy mildew or other soil-borne diseases. Breakdown of soil structure also causes reduced nutrient and water uptake and reduces yield.
Tillage and planting equipment is available to provide systems with varying levels of surface residue for sunflower production. Production systems can range from conventional-till, where the quantity of surface residue covers less than 30% of the soil surface after planting, to no-till, where the quantity of surface residue covers more than 60% of the soil surface after planting.
Conventional-till Production Systems
Conventional-till production systems usually involve two or more tillage operations for breakdown and incorporation of previous crop residues, and weed control. Tillage sequences are determined by the seedbed requirements needed to match planting equipment capabilities and by requirements of soil-applied herbicides.
Conventional tillage systems include the option of using a rotary hoe or harrow for breaking crusted soil surfaces or pre-emergence weed control. The suggested period for utilizing this equipment is prior to sunflower plant emergence.
Proper adjustment of the harrow or rotary hoe will maximize damage to the weeds and minimize injury to the sunflower crop. Conventional tillage systems also include the option of row cultivation for weed control during the early growing season before the sunflowers reach a height too tall for cultivation.
.jpg)
Air Drill Use
Solid-seeded sunflower has become popular with producers in some regions. Air drills commonly are used to plant solid-seeded stands (Figure 13). Advantages include 1) improved utilization of equipment already owned and 2) ease of changing between crops.
Suggested adjustments to the air drill when planting sunflower include: 1) Use the proper metering roller, 2) Slow the metering roller speed, 3) Calibrate the drill. Run through the calibration cycle 10 times and then three additional times to check for consistently metered weights, 4) Recalibrate the drill every time hybrid or seed lot changes, 5) Reduce airflow. Provide the minimum amount of air to move seed and fertilizer to the opener so the seed is not damaged, 6) Don’t place all of your seed in the bin after the drill is calibrated. Place a couple of bags in the bin and run until the low seed light appears. Then place another bag in the hopper and run until the low seed light appears. Calculate the number of acres seeded. If you appear to be planting the correct seeding rate, place one more bag of seed in the hopper and run until the low seed light appears. If this seeding rate is correct, fill the seed bin and plant the rest of the field.
No-till Production Systems
No-till is a production system without primary or secondary tillage prior to, during or after crop establishment that relies heavily on diverse crop rotations. The goal of these production systems is to maintain at least 60% surface cover by crop residue after planting. However, it will depend on the amount of crop residue from the previous crop. Crop residues protect soils from erosion, control weeds, suppress evaporation and improve soil water infiltration.
Planting sunflower in a no-till production system may require the addition of residue managers to move a minimum of crop residue from the seed row so double-disc openers can place seed properly for good seed-to-soil contact. Single-disc openers and narrow-point hoe openers have been used successfully to seed sunflower.
No-till and One-pass Seeding
Sunflower seed should be placed 1.5 to 2 inches deep. In undisturbed silt loam soils, the dry/wet interface usually will be found about 1 inch below the soil surface. In coarse-textured soils, this interface will be deeper. Planting at or just below this dry/wet interface will result in poor and/or uneven germination, resulting in either germinating seed running out of water and dying before the plant has a chance to establish or seed lying in dry soil until adequate rainfall is received.
One-pass seeding operations utilizing high-disturbance openers (sweeps, hoes and narrow points) can produce uneven stands under dry conditions. Seeding depth is more variable with these types of openers, compared with the single-disc style, and moisture conditions will be more variable.
Long, dry periods at planting do occur in North Dakota. Soils will dry below acceptable levels for germination to the depth of the disturbed soil in the seedbed. Uneven germination, emergence, plant stands and plants at different stages of maturity will occur unless adequate moisture is received shortly after planting to rewet the seedbed.
Anhydrous ammonia (Figure 14) sometimes is applied in one-pass seeding operations with openers specifically designed for ammonia application at the time of seeding. If moisture is sufficient and application rates do not exceed 50 pounds per acre, little damage to germinating seed will occur. However, if the seedbed is abnormally dry and/or the soil does not seal properly between the anhydrous ammonia band and the seed, damage to germinating seed will occur.
No-till row planters with row cleaners ahead of double-disc openers are equipped with a liquid or dry fertilizer attachment. These attachments band nitrogen fertilizers separately from the seed band, eliminating injury to the germinating seed.

- Figure 14. This one-pass seeding operation is seeding directly into wheat stubble. Anhydrous ammonia and seed are banded through special openers. (Roger Ashley, NDSU)
.jpg)
Planting Dates
Sunflower may be planted during a wide range of dates. In the northern Great Plains, planting may extend from May 1 until late June. Early maturing hybrids should be selected for late planting or replanting.
Growing conditions during the season will affect yield, oil content and fatty acid composition. High temperatures during seed formation have been identified as the main environmental factor affecting the ratio of linoleic and oleic acid content. Therefore, the optimum planting date will be dependent upon the hybrid and location, as well as anticipated weather conditions during the growing season.
High yield may be obtained from early planting dates, but yield may be reduced by increased pest problems. However, early planting may reduce bird damage and reduced late-season loss from Sclerotinia head rot due to early plant maturity.
Late June plantings often result in lower yields and oil content. In addition, when harvest is delayed by weather, mechanical drying of seed is required, thus adding to production expenses.
The fatty acid profile also is affected by planting date. In a three-year planting date study in southwestern North Dakota, oleic fatty acid content was greatest when the planting date occurred around May 23 and lowest when the planting date was later than June 10.
Row Spacing and Plant Population
Sunflower will perform well in a wide range of plant populations and plant spacing. With the presence of recommended production management practices, seed yield is similar between sunflower seeded in rows and solid seeded (row spacing of less than 20 inches) (Figure 15). Equidistant spacing of seeds should produce a uniform sunflower stand, which makes maximum use of resources, such as water, nutrients and sunlight.
Use seed spacing to achieve the desired plant population. Table 9 assumes seed germination is 90% and a 10% stand reduction will occur between emergence and harvest. The seed spacing must be adjusted with lower or higher germination rates, and thus spacing between seed.
.jpg)
Table 9. Seed spacing required for various populations, assuming 90% germination and 10% stand loss.
Highlighted seed spacings provide nearly equal-distant spacing between plants for a given row spacing and plant population.
Desired seed spacing may be calculated using the following formula:
SS = (6,272,640/RS)/(PP/(GR x SR)
Where:
SS = in row seed spacing in inches
RS = between row spacing in inches
PP = desired plant population at harvest
GR = germination rate as a decimal. For example, if germination is 95%, then germination rate = .95.
SR = stand reduction as a decimal. This reduction is a result of other factors between germination and final harvest population. For example, if a 10% reduction is expected, then 100%–10% = 90%, or .9.
Sunflower plants will compensate for differences in plant population by adjusting seed and head size. As the plant population decreases, seed and head size will increase.
Oilseed hybrids generally are planted at higher populations than nonoilseed hybrids because the size of harvested seed is less important. Plant populations for oilseed sunflower should be between 18,000 and 24,000 plants per acre, with adjustments made for soil type, rainfall potential and yield goal. Nonoilseed sunflower should be planted at populations between 14,000 and 20,000 plants per acre.
Solid-seeded sunflower populations should be at the high end of these ranges. Lower populations are recommended for soils with lower water-holding capacity and if normal rainfall is inconsistent or inadequate. Preharvest dry-down is more rapid in higher plant populations because of the smaller head size. However, higher plant populations may result in increased lodging and stalk breakage.
Proper planting equipment adjustment and operation is one of the most important management tasks in sunflower production. Plateless and cyclo air planters have been used effectively to get good seed distribution. Double-seed drops should be avoided and planter adjustments should be made.
Conventional plate planters will provide good seed distribution by using correct planter plates, properly sized seed and proper seed knockers. Commercial seed companies have plate recommendations for all seed sizes. Grain drills and air seeders may be used for seeding, although uniform depth of planting and seed spacing may be a problem unless proper adjustments and modifications are made.
Postharvest Tillage
After harvest, tillage of sunflower stalks is not recommended because the snow trapping potential is diminished, thereby reducing soil water conservation potential during the winter for the following crop. Also, because of the nature of sunflower residues, a late harvest followed by late fall tillage leaves the soil extremely susceptible to wind and water erosion.
Crop Rotation
Greg Endres
Having a proper rotation sequence with all crops, including sunflower, is important. Crop rotations should include cool-season grass and broadleaf crops, as well as warm-season grass and broadleaf crops. Research in Crookston, Minn., showed that sunflower seed yield was 5% higher after potato, 20% higher after sugarbeet, 8% higher after pinto bean and 17% higher after wheat, compared with sunflower grown after sunflower.
Growers who do not rotate sunflower fields likely will be confronted with one or more of the following yield-reducing problems:
- Disease and disease-infested fields
- Increased insect risk
- Increasing populations of certain types of weeds, including herbicide-resistant weeds
- Increased populations of volunteer sunflowers
- Soil moisture depletion
Therefore, producers have many valid reasons for rotating sunflower fields.
Risks of sunflower disease will be greatly magnified by short sequencing of sunflower in a crop rotation. Sclerotinia or white mold (wilt, stem rot and head rot) is the primary disease concern with a short sunflower rotation.
Rotations of at least three- or four-year spacings between sunflower or other Sclerotinia-susceptible crops (e.g., canola, dry bean, soybean) are recommended to help reduce disease risk. The sunflower disease section in this publication contains specifics on the characteristics and methods of management for each disease.
Crop rotation may help reduce but will not prevent insect problems in sunflower. Proper rotations help reduce populations of insects that overwinter in the soil or sunflower plant residue. Crop rotation will not reduce damage from insects that migrate into an area from other geographic regions or from fields planted to sunflower the previous year that are in proximity to current-season fields.
Rotations recommended for reducing sunflower disease risks also will reduce insect risks. Different patterns of soil moisture utilization are important considerations when planning sunflower rotations.
Rotation of other crops with sunflower can reduce the buildup of many weed species. Also, proper crop rotation increases weed management options, including cultural, mechanical and chemical weed control. Consult records of previous field management to determine if long-residual herbicides that would adversely affect sunflower production were used.
Volunteer sunflower also can become a serious weed problem in other broadleaf crops. For additional details, refer to the weed management section of this publication, herbicide labels and NDSU Extension publication W253, “North Dakota Weed Control Guide.”
Pollination
Gary Brewer
Native sunflowers and the early varieties of sunflower were self-incompatible and required insect pollination for economic seed set and yields. Current hybrids have been selected for and possess high levels of self-compatibility. However, modern hybrids benefit from insect pollination.
The agronomic value of insect pollinating activities to current hybrids varies among hybrids, fields and years. In most sunflower hybrids, seed set, seed oil percentage and seed yields increase when pollinators (primarily bees) are present. Scientific literature indicates that yield could increase as much as 48.8% and oil percentage could increase 6.4% in bee-exposed hybrids. However, despite the increases in yield and oil concentration that occur, the benefit of insect pollination of sunflower often is overlooked.
Although native wild bees are often better pollinators of sunflower than honey bees, the honey bee is the only managed pollinator of sunflower available. However, if pollen sources other than sunflower are nearby, the honey bee will forage sunflower primarily for nectar and will not transfer sunflower pollen efficiently.
Honey bee colonies are placed in seed production fields at a rate of one hive per one to two acres. A bee density of more than 20 bees per 100 heads in bloom is needed to transfer sufficient pollen from the male line to the female sterile line.
Placement of honey bee colonies will depend upon proximity and acreage of competing nectar and pollen sources. With no competition, all honey bee colonies are placed at one end of the target field. With competing nectar and pollen sources, placement of honey bee colonies at 800-foot intervals may be necessary.
Maximum seed yields often require the use of insecticides to protect the crop from insect competitors. Unfortunately, many of the major insect pests of sunflower attack the crop when it is flowering. Thus, insecticides used to control the pest also harm pollinating bees. If pollinator activity is decreased, yield and oil percentage may decline.
The hazards to honey bees can be minimized with adequate communication and cooperation among beekeepers, growers and pesticide applicators. Beekeepers must inform applicators of the location of apiaries and be prepared to move or protect colonies. When insecticide spraying is justified, applicators must make every attempt to notify beekeepers in advance.
Pollinator Safety
Janet Knodel
Flowering sunflowers are very attractive to pollinators, especially bees, and a major source of honey for honey bees in North Dakota. Any insecticide applied during flowering would be deadly for honey bees, native bees and other pollinators. Unfortunately, the timing of insecticide treatments for most sunflower insect pests is during the early flowering stages (R5.1 to R5.4), when insect pest populations are at levels to cause economic loss.
NDSU Extension Entomology recommends spraying insecticides only when insect pests are at the economic threshold levels in sunflower fields. Insecticide treatments should be applied during the early morning or late evening, when most bees are back in the hive. Most insecticides registered in sunflowers are highly toxic to all insects, including bees and other pollinators, so selection of a less toxic insecticide or more pest-specific insecticide is problematic.
The North Dakota Department of Agriculture (NDDA) has apiaries mapped in the state, so pesticide applicators/growers can find where the registered beehives of North Dakota are located and the contact information for that beekeeper. If a field, especially in the flowering stage, has beehives nearby and it needs to be treated with an insecticide for insect pest control, this map can help improve communication among pesticide applicators, growers and beekeepers, and help save honeybees from pesticide poisonings. Please see the NDDA map at https://ole.ndda.nd.gov/map.
Pest Management
Integrated Pest Management
Janet Knodel and Patrick Beauzay
Sunflower can be a high-risk crop because of potential losses from diseases, insects, birds and weeds. These potential risks require that growers follow integrated pest management (IPM) practices. IPM is a sustainable approach to managing pests by combining biological, cultural, physical and chemical tools in a way that minimizes economic, health and environmental risks to maintain pest populations below levels that cause unacceptable losses to crop quality or yield.
The concept of IPM is based on the fact that many factors interact to influence the abundance of a pest. Integration of various management strategies can minimize the number of pests in sunflower and reduce the cost of managing pest populations without unnecessary crop losses. IPM also recommends the judicious use of pesticides only when needed, and suggests ways to maximize effectiveness and minimize impact on nontarget organisms and the environment.
Economic Injury Level and Economic Threshold Levels
One major component of a pest management program is determining when tactics should be implemented to prevent economic loss. Economic loss results when pest numbers increase to a point where they cause crop losses that are greater than the cost of controlling the pest.
The point at which crop value loss equals pest treatment costs is called the economic injury level (EIL). An EIL recognizes that treatment is justified for some pest population levels while other pests are not of economic importance.
An economic threshold (ET) is the level or number of pests at which tactics must be applied to prevent an increasing pest population from causing economic loss. Usually the ET is lower than the EIL. The ET varies significantly among different pests and also can vary during different developmental stages of the crop.
Crop value, yield potential, crop density, cost of control and environmental conditions influence the ET and EIL. Generally, the ET increases as the cost of control increases and decreases as the crop value increases.
Monitoring Pest Population Levels
In general, fields should be evaluated regularly to determine pest population levels. A weekly field check is usually sufficient, but field checks should be increased to two or three times a week if the pest’s population is increasing rapidly or if the number is approaching an economic threshold level.
Pest identification is important because economic thresholds and control measures vary for different pests. In addition, many insects are beneficial and may help reduce numbers of injurious insects. Recognizing which are pests and those that are beneficial is important.
Tools of Integrated Pest Management
IPM tools include many tactics, of which pesticides are only one. These tactics can be combined to create conditions that are least conducive for pest survival. Chemical or biological pesticides are used when pests exceed economic thresholds. Sometimes they are necessary when control is needed quickly to prevent economic loss.
Some of the tools or components of pest management that can be used to reduce pest populations are:
Biological Controls
Beneficial insects
Beneficial pathogens
Host resistance
Cultural Controls
Planting and harvest dates
Crop rotation
Tillage practices
Mechanical/Physical Controls
Temperature
Weather events
Trapping
Chemical Controls
Pesticides
Attractants
Repellents
Pheromones
The following sections provide current information on management of insects, diseases, weeds, birds and other sunflower pests. A growing season calendar shows the major sunflower pest problems and time of occurrence in the northern Great Plains production area (Figure 16).
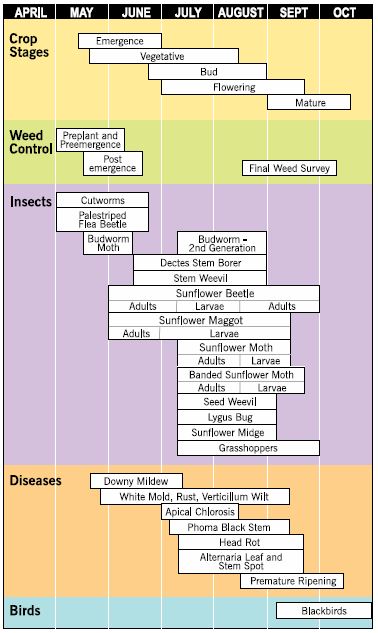
- Figure 16. A growing season calendar indicating time of occurrence of major sunflower pests. (Janet Knodel, NDSU)
Insect Pest Management
Janet Knodel, Patrick Beauzay and Anitha Chirumamilla
Quick Reference Guide to Major Sunflower Insects
The insects in Table 10 are listed in the order that they likely occur throughout the growing season; however, the various insects may or may not appear, depending upon overwintering survival and environmental conditions as the season progresses. The table is intended as a guide to when fields should be scouted for various insect pests of sunflowers.
Table 10. Reference guide to major sunflower insects.
|
Insects |
Description |
Crop Damage |
Economic Threshold |
|
Wireworms |
Adult: bullet-shaped, hard-shelled beetles, brown to black, about Larva: hard, smooth, slender, wirelike worms varying from white, yellow to copper and 1.5 to 2 inches in |
Larva damage crop by feeding on the germinating seed or the young seedling. Damaged plants soon wilt and die, resulting in thin stands or bare spots in the field. Reseeding may be necessary in severe wireworm infestations. Bait trapping and history of wireworm are used to predict infestations. |
Bait trapping – greater than one wireworm per bait station results in a high risk of crop injury. An insecticide seed treatment or a soil insecticide should be used at planting to protect the sunflower from wireworm damage. If no wireworms are found in the traps, risk of injury is low. |
|
Cutworms |
Adult: brown, gray or black with variable markings on forewings, about 1¼ to 1½ inch wing span, depending on species. Larva: gray to brown larva, 0.25 to 1.5 inches in length. |
Appear early spring when plants are in the seedling stage. Larva cut or clip plants, which leads to reduced stands and potential yield losses. Moths and larvae are actively feeding during night, which may make scouting observations difficult during the day. When scouting, look for freshly cut plants and dig 3 inches deep around cut plants to find larvae. |
One larva per square foot or 25% to 30% stand loss. Applied insecticide during the evening when cutworms are active. |
|
Grasshoppers |
Adults: Green, yellow or brown, 0.75 to 2 inches long when mature, depending on species, enlarged hind legs for jumping. Nymphs: similar to adults in general appearance, but are smaller in size, with wing pads. |
Adults and nymphs are defoliators, feeding on green plant material and creating holes on leaves or chewing on heads later in the year. |
Adult: greater than 20 adults per square yard in field margins or eight to 14 adults per square yard in the field. Nymph: greater than 50 nymphs per square yard in field margins, or when greater than 30 nymphs per square yard in the field. |
|
Palestriped flea beetle |
Adult: 1/8 inch long and shiny black, with two white stripes on the back. The hind legs are enlarged for jumping. |
Scout for flea beetles in the spring by visually estimating populations on seedlings or using yellow sticky cards to trap beetles. |
20% of the seedling stand is injured by beetle. |
|
Sunflower beetle |
Adult: reddish-brown head, cream back with three dark stripes and an exclamation mark on each wing cover and body length 0.5 inch. Larva: yellowish green, humpbacked in appearance, 0.35 inch in length. |
Adults appear in early June and larvae shortly thereafter. Adults and larvae chew large holes in leaves. |
Adult: One to two adults per seedling. Larva: 10 to 15 larvae per plant, or when 25% defoliation on the upper eight to 12 leaves (active growing part). |
|
Sunflower bud moth |
Adult: wingspread 0.63 to 0.75 inch, gray brown with two dark transverse bands on forewings. Larva: Cream-colored body (0.33 to 0.4 inch) with a brown head, when mature |
First generation adults appear in late May to mid-June. Second generation adults appear in midsummer. Larvae from the first generation damage terminals and stalks, whereas second generation larvae feed in the receptacle area. |
No scouting method or ET has been developed. |
|
Dectes stem borer |
Adult: pale gray and 5/8 inch in length, with long gray and black banded antennae. Larva: yellowish with fleshy protuberances on the first seven abdominal segments (0.5 inch). |
Adults are present from late June through August. Larvae tunnel and feed in the petioles and stem pith and girdle the base of plants. Stalks often break at the point of larval girdling. |
No scouting method or ET has been developed. |
|
Sunflower stem weevil |
Adult: small (0.19 inch long) gray-brown weevil with white dots on the back. Larva: ¼ inch long at maturity, creamy white with a small, brown head capsule, C-shaped, legless. |
Adults appear in mid- to late June, with larvae in stalks from early July to late summer. Larvae weaken the stem from tunneling, pith destruction and especially by construction of overwintering chambers at the stalk base. A larval infestation of 20 to 25 or more per stalk increases the risk of stalk breakage and lodging (loss of the entire head). |
One adult per three plants in late June to early July. |
|
Thistle caterpillar (Painted lady butterfly) |
Adult: wingspread of 2 inches, upper wing surface brown with red and orange mottling and white and black spots. Larva: brown to black, spiny, with a pale yellow stripe on each side, 1.5 inches in length, when mature |
Adult appear in early to mid-June, with larvae appearing shortly thereafter. Larvae chew holes and skeletonize leaves. |
25% defoliation, provided that most of the larvae still are less than 1.25 inches in length. |
|
Sunflower midge |
Adult: small (0.07 inch), tan, gnatlike insect. Larva: small, 0.09 inch long, cream or yellowish, tapered at front and rear. |
Adult emergence begins in early July. Larvae feed underneath bracts of the head and at the base of the seeds, causing shrinkage and distortion of heads. |
No scouting method or ET has been developed. |
|
Red sunflower seed weevil |
Adult: rusty red and about 0.12 inch long. Larva: cream-colored, legless and C-shaped. |
Adults appear in late June to early July. Treat for red sunflower seed weevil at R5.1 to R5.4. Larvae feed in seeds from mid to late summer. |
Oilseed sunflowers - calculated ET of four to eight adult red sunflower weevils per head. Confection sunflowers - one per head. |
|
Gray sunflower seed weevil |
Adult: gray weevil and about 0.14 inch long. Larva: cream-colored, legless and C-shaped. |
Field scouting should begin at bud stage R2. Seeds infested by the gray seed weevil lack a kernel and, due to their light weight, the seeds may be lost during the harvesting. |
No ET has been developed. If fields require an insecticidal treatment, they need to be sprayed in early bud stage to target adults before egg laying. |
|
Sunflower moth |
Adult: gray body is 0.38 inch long, with 0.75 inch wingspread. Larva: brown head capsule with alternate brown and cream lines running longitudinally, 0.75 inch in length. |
Adults are migratory and usually appear in early to mid-July. Larvae tunnel in seeds and back of the head from late July to late August. |
One to two adults per five plants at onset of bloom. |
|
Banded sunflower moth and Arthuri sunflower moth |
Adult: small 0.25-inch straw-colored moth with brown triangular area on forewing. (Arthuri sunflower moths are lighter – white to gray). Larva: in early stages, off-white, changing to red and then green, 0.44 inch in length when mature. |
Field scouting should be conducted in the late bud stage (R3), usually during mid-July. Adults appear about mid-July to mid-August. Larvae feed on seeds from mid-July to mid-September. |
See banded sunflower moth and Arthuri sunflower moth section for calculating thresholds for eggs and adult moths. |
|
Lygus bug |
Adult: small (0.2 inch in length), cryptically colored insects with a distinctive yellow triangle or “V” on the wings and vary in color from pale green to dark brown. Nymph (immature stages): usually green and similar in appearance to the adults, but lack wings. |
Adults and nymphs have piercing/sucking mouthparts that feed on the kernel tissue, causing tissue destruction called brown spot. This results in a bitter taste to the seeds, reducing their quality. |
For confection sunflowers only: One Lygus bug per nine heads. Two insecticide sprays are bloom, followed by a second treatment seven days later. |
In the major sunflower producing areas of the Dakotas, Minnesota and Manitoba, approximately 16 species of sunflower insects can cause plant injury and economic loss, depending on the severity of infestation. However, during any one growing season and based on geographical location, only a few species will be numerous enough to warrant control measures. The sunflower insects of major importance in the northern Great Plains have been sunflower midge, Contarinia schulzi Gagne; sunflower stem weevil, Cylindrocopturus adspersus (LeConte); red sunflower seed weevil, Smicronyx fulvus LeConte; and the banded sunflower moth, Cochylichroa hospes Walsingham.
Lygus bugs have been an economic problem for the confection and hulling sunflower seed market. Populations of the Dectes stem borer, Dectes texanus LeConte, have been increasing in North and South Dakota.
Infestation of sunflower insects must be monitored regularly, usually weekly, to determine the species present and if populations are at economic thresholds. Furthermore, proper timing of insecticidal treatment is essential to maximize control.
Sunflower pests are not distributed evenly throughout a field, and fields should be checked in several locations. Some insect pests, such as sunflower midge, red sunflower seed weevil and banded sunflower moth, are concentrated near the edge of a field. At least five sites per 40-acre field should be monitored to collect good information on the extent of a pest infestation.
Sampling sites should be at least 75 feet in from the field margin to determine whether an entire field or a portion of the field requires treatment. In some cases when infestations occur primarily along field margins, delineating those and treating as little of the field as needed to provide economic control may be possible. In most cases, 20 plants per sampling site should be examined by walking a Z or X pattern in the field (Figure 17).
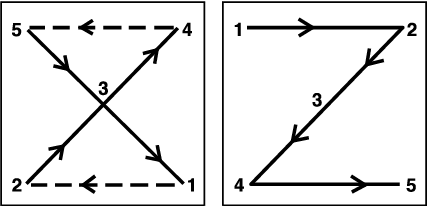
- Figure 17. The X and Z scouting patterns.

Wireworms
Species: various
Description: Wireworm larvae (Figure 18) are hard, smooth, slender, wirelike worms varying from 1.5 to 2 inches in length when mature. They are a yellowish white to a coppery color with three pairs of small, thin legs behind the head. The last body segment is forked or notched.
Adult wireworms (Figure 19) are bullet-shaped, hard-shelled beetles that are brown to black and about 0.5 inch long. The common name “click beetle” is derived from the clicking sound that the insect makes when attempting to right itself after landing on its back.
.jpg)
Life cycles: Wireworms usually take three to four years to develop from egg to an adult beetle. Most of this time is spent as a larva. Generations overlap, so larvae of all ages may be in the soil at the same time. Wireworm larvae and adults overwinter at least 9 to 24 inches deep in the soil. When soil temperatures reach 50 to 55 F during the spring, larvae and adults move nearer the soil surface.
Adult females emerge from the soil, attract males to mate, then burrow back into the soil to lay eggs. Females can re-emerge and move to other sites, where they burrow in and lay more eggs. This behavior results in spotty infestations throughout a field. Some wireworms prefer loose, light and well-drained soils; others prefer low spots in fields where higher moisture and heavier clay soils are present.
Larvae move up and down in the soil profile in response to temperature and moisture. After soil temperatures warm to 50 F, larvae feed within 6 inches of the soil surface. When soil temperatures become too hot (greater than 80 F) or dry, larvae will move deeper into the soil to seek more favorable conditions. Wireworms inflict most of their damage in the early spring, when they are near the soil surface. During the summer months, the larvae move deeper into the soil. Later as soils cool, larvae may resume feeding nearer the surface, but the amount of injury varies with the crop.
Wireworms pupate and the adult stage is spent within cells in the soil during the summer or fall of their final year. The adults remain in the soil until the following spring.
Damage: Wireworm infestations are more likely to develop where grasses, including grain crops, are growing. Wireworms damage crops by feeding on the germinating seed or the young seedling (Figure 20). Damaged plants soon wilt and die, resulting in thin stands (Figure 21). In a heavy infestation, bare spots may appear in the field and reseeding is necessary.

- Figure 20. Wireworm feeding injury in sunflower seedling.(Patrick Beauzay, NDSU)
.jpg)
Scouting method: Decisions to use insecticides for wireworm management must be made prior to planting. No rescue treatments are available for controlling wireworms after planting. Producers have no easy way to determine the severity of infestations without sampling the soil. Infestations vary from year to year. Considerable variation may occur within and between fields.
Sometimes the history of a field is a good indicator, especially if wireworms have been a problem in previous seasons. Also, crop rotation may impact population levels.
Two sampling procedures are available. One procedure relies on the use of a bait mixture with equal proportions of corn-wheat seed, placed in the soil, which attracts the wireworms to the site (Figure 22). The other involves digging and sifting a soil sample for the presence of wireworms.
- Figure 22. Wirewormbait station.(Extension Entomology)

Economic threshold: If the average density is greater than one wireworm per bait station, the risk of crop injury is high and an insecticide seed treatment or a soil insecticide should be used at planting to protect the sunflower. If no wireworms are found in the traps, risk of injury is low; however, wireworms still may be present but were not detected by the traps. When digging soil samples, 12 or more wireworms in 50 3-inch by 3-inch samples, is likely to result in damage to sunflower.
Management: Seeds should be treated with an approved insecticide for protection of germinating seeds and seedlings. Increasing the seeding rate to compensate for wireworm stand loss is another management tactic.
Cutworms
Species:
Darksided cutworm, Euxoa messoria (Harris)
Redbacked cutworm, Euxoa ochrogaster (Guenee)
Dingy cutworm, Feltia jaculifera (Walker)
Description: Darksided cutworm — Forewings of the adult darksided cutworm are usually light, powdery and grayish brown with indistinct markings (Figure 23). The larvae are pale brown dorsally and white on the ventral areas (Figure 24). Sides have numerous indistinct stripes. At maturity, they are about 1.25 to 1.5 inches long and 0.19 inch wide.
.jpg)
Redbacked cutworm — The forewings of the adult redbacked cutworm are reddish brown with characteristic bean-shaped markings (Figure 25). The larvae are dull gray to brown with soft, fleshy bodies and may be 1 to 1.25 inches long when fully grown (Figure 26). Larvae can be distinguished by two dull reddish stripes along the back.
Dingy cutworm — Forewings are dark brown with bean-shaped markings as in the redbacked cutworm adults (Figure 27). Hind wings in the male are whitish with a broad, dark border on the outer margin; in the female they are uniform dark gray. The larvae have a dull, dingy, brown body mottled with cream color. The dorsal area is pale with traces of oblique shading (Figure 28).

- Figure 25. Adult –Redbacked cutworm. (Gerald Fauske, NDSU)

- Figure 26. Larva – Redbacked cutworm. (John Gavloski, Manitoba Agriculture, Food and Rural Initiatives)
.jpg)


- Figure 27. Adult – Dingy cutworm. (Gerald Fauske, NDSU)

- Figure 28. Larva – Dingy cutworm. (John Gavloski, Manitoba Agriculture, Food and Rural Initiatives)
.jpg)
.jpg)
Life cycles: The female darksided and redbacked cutworm moths deposit eggs in the soil in late July and early August. The eggs remain dormant until the onset of warm weather the following spring. Larvae of both species emerge from late May to early June. They continue to feed and grow until about the end of June. When fully grown, larvae pupate in earthen cells near the soil surface. The pupal period lasts about three weeks. Both species have one generation per year.
The adult dingy cutworms emerge in August and are active until mid-October, with peak activity in September. Eggs are deposited in plants in the Compositae family in the fall. Larvae develop to the second or third instar in the fall and overwinter in the soil. Pupation occurs in the spring to early summer. One generation of this species is produced per year.
Damage: Cutworm damage normally consists of crop plants being cut off from 1 inch below the soil surface to as much as 1 to 2 inches above the soil surface. Young leaves also may be severely chewed as a result of cutworms (notably darksided cutworm) climbing up to feed on the plant foliage.
Most cutworm feeding occurs at night. During the daytime, the cutworms usually will be just under the soil surface near the base of recently damaged plants. Wilted or dead plants frequently indicate the presence of cutworms. Cut-off plants may dry and blow away, leaving bare patches in the field as evidence of cutworm infestations.
Scouting method: Sampling should begin as soon as sunflower plants emerge, and fields should be checked at least twice per week until approximately mid-June. The Z pattern should be used in scouting fields for cutworms, with sampling points one and two near the margin as indicated in Figure 17.
Stand reduction is determined by examining 100 plants per five sampling sites for a total of 500 plants. A trowel or similar tool should be used to dig around damaged plants to determine if cutworms are present because missing plants in a row do not necessarily indicate cutworm damage (damage may be caused by a defective planter, rodents or birds).
The Z pattern should be used again to determine cutworm infestation level by examining five 1-square-foot soil samples per site (in the row) for a total of 25 samples.
Economic threshold: One larva per square foot or 25% to 30% stand reduction.
Management: Several different insecticides are registered for cutworm control in sunflower. Optimal timing of a foliar application of an insecticide is during the evening when cutworms are most actively feeding. In most situations, a broadcast foliar application of an insecticide provides quick control of surface feeding cutworms.
Grasshoppers
Species: Melanoplus spp. (Family Acrididae)
Description: Adult grasshoppers are approximately 0.75 to 2 inches long, depending on the species. The two-striped grasshopper (Figure 29) is a common grasshopper of field crops. Adults are yellow-brown with two light yellow stripes running from the head to the end of the wings.
The Immature grasshopper is called a nymph (Figure 30). Nymphs are similar to adults in general appearance but are smaller and have wing pads instead of wings.

- Figure 29. Adult –two-striped grasshopper.(Janet Knodel, NDSU)
.jpg)
Life cycle: In the northern Plains, grasshopper egg hatch normally begins in late April to early May, with peak hatch occurring into mid-June. Typically, egg hatch will approach completion by late June.
Most grasshoppers emerge from eggs deposited in uncultivated ground. A female grasshopper produces seven to 30 egg masses per season. Nymphs hatch from the eggs and have five to six nymphal stages. The beginning of bloom in common lilac has been used as an indicator of when grasshopper hatch is underway. Grasshoppers have one generation per season and development takes about 40 to 60 days from egg to adult.
Adults of crop-damaging grasshopper species become numerous in mid-July and continue into fall. Egg laying activity usually begins in late July and continues through fall. Eggs are deposited in soil in a variety of noncrop areas, including ditches, fence rows, shelter belts, weedy areas, hay lands and alfalfa.
Damage: Adults and nymphs are defoliators, feeding on green plant material and creating holes on leaves or chewing on heads later in the year. High populations (outbreaks) may result in yield loss and a delay in maturity (Figure 31).

- Figure 31. Severely defoliated sunflower field from grasshopper feeding. (Aaron Hargens, South Dakota State University)
.jpg)
Scouting method: Growers should scout for grasshopper nymphs in the spring along field margins adjacent to noncrop sites where the egg laying occurred in the fall. After the small grain harvest, adult grasshoppers often will move in large numbers into sunflower and other row crops, so frequent scouting is needed. A sweep net often is used to collect fast-moving grasshoppers in field ditches next to fields. However, sunflowers are tall and difficult to sweep effectively. Therefore, visual counts on the number of adult grasshoppers per square yard from multiple locations in the field are required.
Economic threshold: The threatening rating is considered the nominal economic threshold for grasshoppers (Table 11). For example, grasshopper control is advised whenever 50 or more small nymphs per square yard can be found in adjacent, noncrop areas, or when 30 or more nymphs per square yard can be found within the field. When 20 or more adults per square yard are found in field margins or eight to 14 adults per square yard are occurring in the crop, treatment would be justified. Because estimating the number of grasshoppers per square yard is difficult when population densities are high, pest managers can count grasshoppers collected from four 180-degree sweeps when using a 15-inch sweep net and use that value as an estimate for the number of adult (or nymph) grasshoppers per square yard.
Table 11. Levels of grasshopper infestation and action threshold.
|
Rating |
Nymphs per square yard |
Adults per square yard |
|||
|
Margin |
Field |
Margin |
Field |
||
|
Light |
25-35 |
15-23 |
10-20 |
3-7 |
|
|
Threatening |
50-75 |
30-45 |
21-40 |
8-14 |
|
|
Severe |
100-150 |
60-90 |
41-80 |
15-28 |
|
|
Very severe |
200+ |
120 |
80+ |
28+ |
|
Management: Well-timed applications of insecticides are used commonly when grasshopper populations exceed the economic threshold level. Grasshopper infestations are often the heaviest on field margins. Outbreaks usually are preceded by several years of hot, dry summers and warm falls, allowing populations to increase slowly. Early planted sunflowers will mature earlier and the risk of late-season migration of adult grasshoppers into these fields should be lessened, thus reducing late season crop damage.
Palestriped Flea Beetle
Species: Systena blanda (Melsheimer)
Description: The adult is about 0.13 inch long and shiny black, with two white stripes on the back. The hind legs are enlarged and modified for jumping (Figure 32).

- Figure 32. Adult – Palestriped flea beetle. (James Kalisch,University of Nebraska)
.jpg)
Life cycle: The life cycle of palestriped flea beetles on sunflower fields is poorly understood. However, the adult flea beetles seem to overwinter in the field under soil clods, field debris and crop residues. They become active again in the spring, perhaps feeding first on alfalfa and weeds before moving to and feeding on sunflower seedlings in June. They have been observed feeding on sunflower through July. Palestriped flea beetles have a wide host range, which includes various weeds, potato, tomato, carrot, corn, oat, pea, beans, strawberry, watermelon, grape and pumpkin. Palestriped flea beetles are considered an important pest of commercially grown vegetables in some areas of the U.S. Recently, palestriped flea beetles have been observed delaying regrowth of alfalfa and also were observed feeding on soybean seedlings in eastern South Dakota.
Damage: Palestriped flea beetles chew on the cotyledons, leaves and hypocotyls of sunflower seedlings, causing them to wilt and die. Injured leaves become riddled with holes, giving them a “lacey” appearance (Figure 33). The sunflower plant is most sensitive to palestriped flea beetle injury from seedling emergence (VE) through the four-leaf stage (V4). Significant stand losses may result from heavy feeding injury by the palestriped flea beetles.
Scouting method: Survey methods can include using yellow sticky cards placed close to the ground (Figure 34). Visual observations of beetles on seedlings also can aid in estimating populations and feeding injury levels. Palestriped flea beetles move very fast and are hard to count directly on the seedlings or catch with an insect net.

- Figure 33. Damaged sunflower leaves by palestriped flea beetle. (Michael Catangui)

Economic threshold: Control is recommended when 20% of the seedling stand is injured and at risk for loss due to palestriped flea beetle feeding. This economic threshold is a guideline based on published hail injury data that predict potential yield loss relative to seedling stand loss.
Management: Palestriped flea beetles are hard to control with foliar insecticides; research has shown that treatments may provide up to 75% control of adults. An insecticide seed treatment can provide adequate protection against flea beetles only early in the season.
Sunflower Beetle
Species: Zygogramma exclamationis (Fabricius)
Description: The sunflower beetle is associated exclusively with sunflower. Adults (Figure 35) closely resemble adult Colorado potato beetles and may be confused with potato beetles. However, sunflower beetles are smaller and do not feed on potatoes, and Colorado potato beetles do not feed on sunflower. The head of the adult is reddish brown and the thorax (area between head and abdomen) is pale cream-colored with a reddish-brown patch at the base. Each front wing cover is cream-colored and has three dark stripes that extend its length. A shorter lateral stripe ends at the middle of the wing in a small dot that resembles an exclamation point. The beetle is 0.25 to 0.5 inch long and 0.1 to 0.2 inch wide. Eggs are about 1/16 inch long, cigar-shaped and cream yellow. Sunflower beetle larvae are yellowish green with a brown head capsule and humpbacked in appearance. Newly hatched larvae are about 1/16 inch long and will reach a length of about an inch when fully developed (Figure 36).


Life cycle: The sunflower beetle has one generation per year. The adults overwinter in the soil, emerging in late May or early June. Shortly after emergence, the beetles begin to feed, mate and lay eggs singly on stems and undersides of leaves. Adults live for about 8½ weeks and lay eggs for a six- to seven-week period. Each female lays approximately 850 eggs, with a range of 200 to 2,000 eggs. Eggs hatch into larvae in about one week (Figure 37). The larva has four instars, which feed and are present in fields for about six weeks. When mature, the larva enters the soil to pupate in earthen cells. The pupal stage lasts from 10 days to two weeks. Adults of the new generation emerge and feed for a short period on the bracts of the sunflower head or on the uppermost leaves of the plant before re-entering the soil to overwinter.
Damage: With the neonicotinoid seed treatment labeled on sunflowers in the early 2000s, sunflower beetle populations have declined rapidly and are rarely economic in sunflowers now.
Adult sunflower beetles damage plants soon after they emerge from overwintering. Damage to cotyledons is generally slight, but the first true leaves may be severely damaged or completely consumed. Fields may be severely defoliated if beetles are numerous.
Adults feed predominately on leaf margins while larvae feed on the entire leaf surface. When larvae are numerous, damaged leaves take on a lacy appearance. Most larval feeding occurs at night, and adults will feed during the day. During the daytime, larvae typically rest in the terminal growth area, where they are easily found in leaf axils and flower buds. If larval feeding is severe, defoliation can reduce yield due to poor seed set or fill.
The late summer generation of emerging sunflower beetle adults and late-maturing larvae rarely causes economic damage to the sunflower crop. However, in some cases, they have been abundant enough to cause feeding injury on late-planted sunflower.
Scouting method: Sampling sites should be at least 75 to 100 feet from the field’s margins when determining if an entire field should be treated. Adults and/or larvae should be counted on 20 plants at each of five sampling sites along an X pattern for a total of 100 plants. The average number of adults and/or larvae per plant then should be determined.
The average percent defoliation of plants is determined when damage is evident in the field by examining 20 plants per five sampling sites for a total of 100 plants (Figure 38).
- Figure 38. Percent defoliation of sunflower leaves. (Extension Entomology)

Economic threshold: In the seedling stage, one to two adults per seedling is the recommended economic threshold. As sunflower plants develop, they can tolerate more feeding damage. For larvae, the treatment threshold is when populations reach 10 to 15 larvae per plant, or when approximately 25% defoliation occurs on the upper eight to 12 leaves (active growing part). Treatment is advised if defoliation reaches the 25% to 30% level at the late vegetative and early bud stages and it appears (based on larval size of less than ¼ inch) that more defoliation will occur on the actively growing part of sunflower plant. However, if defoliation is 25% and the majority of larvae are about 1/3 inch long, they have reached maturity and soon will stop feeding. Then treatment is not justified.
Management: Insecticide seed treatments and foliar insecticides are effective in reducing spring populations of the adult sunflower beetle. Application of a foliar insecticide is recommended only when beetle populations have reached an economic threshold level in a field. Insecticides are effective in preventing economic loss when applied to actively feeding adults and/or larvae. Adult and larval populations of sunflower beetles decrease as the planting date is delayed. Defoliation also is lower at the later planting dates. As a result, delayed planting is effective in preventing yield reductions caused by sunflower beetle feeding, but may make fields more attractive to later season insects, such as the red sunflower seed weevil. Spring or fall cultivation does not reduce the overwintering populations of sunflower beetle adults or influence the pattern of emergence from the soil during the spring and summer.
Sunflower Bud Moth
Species: Suleima helianthana (Riley)
Description: Sunflower bud moths have a wingspread of about 0.63 inch. Each gray-brown forewing has two dark transverse bands (Figure 39). One band extends across the middle of the wing and the second band is near the wing tip. The larva has a dark head capsule with a smooth, cream-colored body and is 0.31 to 0.43 inch at maturity (Figure 40).
.jpg)
Life cycle: Two generations of sunflower bud moth are produced per year in the northern Great Plains. Adults emerge from overwintering pupae during the last week of May to mid-June.
A few days after adult emergence, eggs are deposited on the terminals of immature sunflower or on the receptacle of mature sunflower. Eggs also are deposited in leaf axils. The hatched larvae begin tunneling into the sunflower plant. The initial infestation in mid-June is characterized by an entrance hole surrounded by black frass, or insect excrement.
Mature larvae pupate within the sunflower plant. Pupae move to the opening of the entrance holes formed in the stem or head tissue so that adults can emerge easily.
The second-generation adults appear in July and August. Infestation by the second-generation larvae is not economically important.
Damage: In early planted sunflower, 65% to 85% of the infestations occur in the stalks. In late-planted sunflower, most infestations occur in the pith areas of the head.
Up to 4,000 larvae per acre have been reported in North Dakota and 24,000 larvae per acre have been reported in Texas. Despite these high populations, economic loss due to this insect has been minimal. The only time yield loss is noticeable is when larvae burrow into unopened buds, preventing proper head development. The larvae normally do not feed on developing seeds but confine feeding activities to the fleshy part of the head. Yield losses are generally not economically significant, although injury by the larva produces malformations in the head and stalk (Figure 41).
Scouting method: A field monitoring scheme for this insect has not been established because it is not of economic significance.
Economic threshold: None established.
Management: Insecticide use has not been justified for control of sunflower bud moth.

- Figure 41. Sunflower head damaged by sunflower bud moth. (Janet Knodel, NDSU)
.jpg)
.jpg)
Dectes Stem Borer
Species: Dectes texanus LeConte
Description: The adult is pale gray and 0.6 inch in length, with long gray and black banded antennae (Figure 42) Eggs are about 0.1 inch long and elongate, and turn dark yellow prior to hatch. Mature larvae are yellowish and 0.3 to 0.5 inch in length. Larvae bear fleshy protuberances on the first seven abdominal segments (Figure 43).
.jpg)
Life cycle: Adults appear in mid-June to early July in the southern Plains. Emergence continues through August, with 50% emerged by mid-July in Texas. Eggs are laid four to eight days after mating and eggs are deposited singly in leaf petioles. Approximately 50 eggs are laid per female, with about one-third viable. Eggs hatch in six to 10 days. Larvae tunnel and feed in the petioles and stem pith and finally move to the base of the plant to overwinter. Larvae develop through six instars. In late summer, the mature larvae girdle the inside of the lower stalk or root crown, move below the girdle and pack frass into the tunnels. Stalks often break at the point of girdling, leaving the larva protected in its frass-packed tunnel during the winter. Larvae are cannibalistic and stalks usually harbor only a single larva, even though several may have hatched in a stalk. This insect has one generation per year. Host plants include sunflower, soybean, ragweed and cocklebur.
Damage: Plant damage due to adult feeding appears to be insignificant because the scars do not penetrate the cortex nor encircle the stalk. Larval feeding is apparent when stalks lodge at the point of the girdle, about 2.5 to 3.5 inches above the soil surface.
Scouting method: None has been developed.
Economic threshold: None has been established.
Management: Recent National Sunflower Association surveys indicate that populations of Dectes stem borer are increasing in South Dakota and North Dakota. In the southern Plains, later planting dates and fall or winter tillage have reduced sunflower infestations by this pest. Perennial sunflower species are resistant to stalk infestation, indicating the possibility of breeding hybrids resistant to the Dectes stem borer. Insecticides are ineffective against larvae in sunflower and were determined to be impractical against adults because of the extended emergence period. When larvae are present in the stalks, plants do not always lodge. Utilizing lower plant populations that encourage thicker stalks may help reduce damage from lodging. If fields are suspected of being infested, prompt harvesting will limit losses from lodging.
Sunflower Maggots
Species:
Sunflower receptacle maggot, Gymnocarena diffusa (Snow)
Sunflower maggot, Strauzia longipennis (Wiedemann)
Sunflower seed maggot, Neotephritis finalis (Loew)
Description: The adult forms of all three sunflower maggots (flies) have wings with a distinct brown or yellowish-brown pattern. The name “picture-wing fly” has been given to flies of this type. While all three fly species are similar in appearance, they do have distinguishing differences.
Gymnocarena diffusa – This species is the largest of the three, with a body about 0.4 inch long and a wingspan of approximately 0.75 inch (Figure 44). The eyes of this species are bright green and the wings have a yellowish-brown and somewhat mottled appearance. G. diffusa larvae attain a length of nearly 0.31 inch at maturity. The larva tapers from the front to rear and is yellowish white (Figure 45).
Strauzia longipennis – Adults of this species have a wing spread of about 0.5 inch and a body 0.25 inch long (Figure 46). The wings bear broad, dark bands that form a fairly distinct F-shaped mark near the tips. The larva of S. longipennis is creamy white, headless and legless, as are the larvae of the other two species (Figure 47). The larva tapers slightly at both ends and attains a length of about 0.28 inch at maturity.
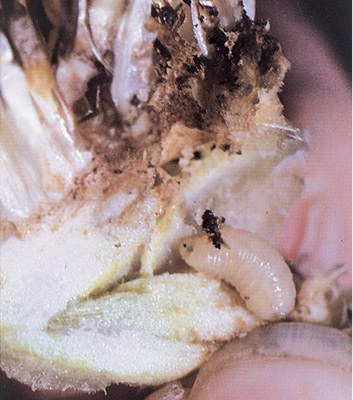
- Figure 45. Larva –Sunflower receptacle maggot.(Extension Entomology
.jpg)
.jpg)
.jpg)

Neotephritis finalis – This sunflower maggot is the smallest of the three species, with the adult having a body length of about 0.25 inch and a wing span of approximately 0.28 inch (Figure 48). The wings have a brown lacelike appearance. N. finalis larvae attain a length of 0.19 inch at maturity. The small, brown pupa of N. finalis is found in sunflower buds and the face of the flowers, usually surrounded by a small number of damaged florets (Figure 49).


- Figure 49. Pupa – Sunflower seed maggot. (Patrick Beauzay, NDSU)
Life cycles: Adults of G. diffusa emerge in late June to early July after sunflower buds reach 2 to 4 inches in diameter. Eggs are laid on the bracts of the developing sunflower heads. Egg laying occurs from mid-July through August. The hatched larvae tunnel into the spongy tissue of the receptacle. Damage to the head is negligible. After 30 days, the mature larvae cut a small emergence hole on the underside of the receptacle and drop into the soil to pupate. Overwintering pupae are found about 7.5 inches deep in the soil by August or early September. Some larvae will pupate in the sunflower head. Only one generation per year occurs in North Dakota.
Strauzia longipennis has one generation per year. This insect overwinters as a larva in plant debris in the soil. Pupation and adult emergence are completed in early June. Females lay eggs in stem tissue of young sunflower, and larvae feed in the pith tissue for much of the growing season.
Unlike the other two species of sunflower maggots, two complete generations per year of N. finalis occur in North Dakota. Adults of N. finalis of the first generation emerge during the first week of July and the second generation during mid-August. About 27 eggs per female are laid on the corolla of incompletely opened sunflower inflorescences. Adult flies can live for 78 days. Larvae feed for 14 days. The first generation of N. finalis pupates in the head; the second generation overwinters in the soil as pupae.
Damage: Damage by sunflower maggots has been negligible, except for N. finalis.
The maggots of G. diffusa feed on the spongy receptacle tissue of the sunflower head and feeding may cause partially deformed heads. Larvae do not feed on developing seeds.
The magnitude of damage to sunflower seeds by N. finalis larvae depends largely on the stage of larval and seed development. Seed sterility occurs when newly hatched larvae tunnel into the corolla of young blooms. Observations indicate that a single larva feeding on young flowers will tunnel through 12 ovaries. In some cases, deformed heads develop from severe seed losses (Figure 50). Mature larvae feeding on older sunflower heads will destroy only one to three seeds.
While infestation levels of S. longipennis occasionally have reached nearly 100%, damage from larval feeding is usually light. Part of a commercial sunflower field next to a grassed waterway or other water source sometimes supports a higher than usual infestation. Under these conditions, high larval numbers of eight to 10 per stalk may be found and stalk breakage can occur. Stalk breakage of up to 30% of the plants has been recorded.

- Figure 50. Deformed head from sunflower seed maggots tunneling through head and feeding on seeds.(Janet Knodel, NDSU)
.jpg)
Scouting method: A scouting method has not been developed for sunflower maggots.
Economic threshold: None has been established.
Management: Insecticide use generally has not been justified for control of any of the sunflower maggots. However, populations of N. finalis have increased and caused significant seed losses due to deformed heads. Research on N. finalis determined that insecticides, even multiple applications, were ineffective against adults, probably due to the wide emergence period. Later planted sunflower, in early June, had less head damage by N. finalis than early planted sunflower, mid- to late May.
Sunflower Stem Weevil
Species: Cylindrocopturus adspersus (LeConte)
Description: Adult sunflower stem weevils are about 0.2 inch long and grayish brown, with varying-shaped white spots on the wing covers and thorax (Figure 51). The snout, eyes and antennae are black. The snout is narrow and protrudes down and backward from the head. Eggs are deposited inside the epidermis of sunflower stems and are very small, oval and yellow, making them difficult to see. The larvae are 0.25 inch long at maturity, legless and creamy white with a small, brown head capsule (Figure 52). They are normally in a curled or C-shaped position within the sunflower stalk. Pupae are similar to the adult in size and creamy white.

- Figure 51. Adult – Sunflower stem weevil.(Extension Entomology)

Life cycle: Only one generation occurs per year. Larvae overwinter in sunflower stalks and crown roots and pupate in the spring, and adults emerge in mid to late June, feeding on the epidermal tissue of the sunflower foliage and stem. This feeding does not affect plant vigor. Mating occurs soon after emergence of adults. Just prior to egg laying, females descend to the lower portion of the plant to deposit eggs individually in the stem tissue. Approximately 50% of oviposition occurs by mid-July. Upon hatching in early July, the first instar (larval growth stage) larvae feed on subepidermal and vascular tissue. Feeding is concentrated in the pith tissue as the larvae develop to third and fourth instar stages. By the last week in August, the larvae descend while feeding to just above the soil surface. A chamber is constructed in the stem, and the weevil overwinters there as a fifth instar larva. Pupation of the overwintering larva occurs the following year in early June.
Damage: Adult sunflower stem weevil feeding causes minor damage to the stem and leaf tissue of the plant. More importantly, adult weevils have been implicated in the epidemiology of the sunflower pathogen Phoma black stem (Phoma macdonaldii) and charcoal stem rot (Macrophomina phaseolina)
Larval injury can cause the stem to weaken from tunneling, pith destruction and especially by construction of overwintering chambers at the stalk base. At larval infestations of 20 to 25 or more per stalk, the plants run a risk of stalk breakage and loss of the entire head. The risk of breakage is greatest when plants are mature and under drought stress and/or during periods of high winds. The breakage typically occurs at or slightly above the soil line, in contrast to breakage attributed to a stalk disease, which normally occurs farther up on the stalks.
Scouting methods: Field monitoring for sunflower stem weevils to estimate population size is important. However, adults are difficult to see on the plants due to their small size, cryptic color and “play dead” behavior. They are inactive on the plant or fall to the ground when disturbed and remain motionless. Adults can be found on both surfaces of the leaves, the lower portions of the stem, in leaf axils, within the dried cotyledons or in soil cracks at the base of the sunflower plant. Yellow sticky traps were unsuccessful in relating captured adult numbers to larval infestations.
Sampling for the larval stage is difficult because they develop totally within the sunflower plant. The only method for detecting the presence of larvae is to split the sunflower stem, a time-consuming process.
Field scouting for adults should begin when plants are in the eight- to 10-leaf stage (V8 to V10) or late June to early July, and continue until mid-July. Select sampling sites 70 to 100 feet in from the field margin. Count the number of adults on five plants at five randomly selected sampling sites throughout the field for a total of 25 plants. Calculate the average number of weevils per plant. Use an X pattern to space sample sites throughout the entire field. When scouting for stem weevils, approach plants carefully and slowly to avoid disturbing the adults.
Economic threshold: Average field counts of one adult sunflower stem weevil per three plants can result in damaging larval densities of more than 40 larvae per stalk at the end of the season.
Management: Insecticidal treatment, if needed based on field counts, should be initiated in late June or early July before significant egg laying has occurred. Cultural control tactics, including delayed planting, altered plant population and tillage, are useful for managing the sunflower stem weevil. Delayed planting of sunflowers until late May or early June has been effective in reducing densities of larvae in the stem. Reducing plant population results in an increased stalk diameter and, as a result, decreases damage from lodging. Combinations of disking to break up stalks and moldboard plowing to bury them at a depth of 6 inches can increase larval/pupal mortality and severely impact the emergence of adult stem weevils. Otherwise, larvae/pupae are physically protected in the woody stalks. Survival is affected only by performing both operations. Greenhouse and field experiments have shown resistance to feeding, oviposition and larval development in many native species of sunflower.
Black Sunflower Stem Weevil
Species: Apion occidentale (Fall)
Description: Adults are black and only 0.1 inch long from the tip of the snout to the tip of the abdomen (Figure 53). The snout is very narrow and protrudes forward from the head, which is small in relation to the rather large, almost globose body. Larvae of A. occidentale are very similar in appearance to C. adspersus, except they are only 0.1 to 0.12 inch long at maturity and yellowish (Figure 54).

- Figure 53. Adult – Black sunflower stem weevil. (Extension Entomology)

- Figure 54. Larva – Black sunflower stem weevil. (Extension Entomology)
.jpg)
Life cycle: Apion occidentale overwinters as an adult in soil, plant residue, sod and weed clusters and begins to emerge and feed on volunteer sunflower as soon as the plants reach the early seedling stage. Females deposit eggs under the epidermis of the stem or leaf petioles. Larvae emerging from these eggs tunnel in the pith area of the stem, pupate and emerge as adults in early August. Little or no adult activity is observed for about two weeks in late July and early August. Black sunflower stem weevil adults emerging in August also feed on the leaves and stems of the plant, but as the plant matures and the leaves begin to die, the adults move under the bracts of the sunflower head, where they can be observed feeding until the plants are harvested.
Damage: Adult feeding injury generally is considered as insignificant. Like the sunflower stem weevil, the black sunflower stem weevil is suspected of vectoring Phoma black stem disease in sunflower fields. In situations of extremely high populations feeding on seedling sunflowers, stand loss has occurred. However, in most cases, populations are too low to cause economic damage and stalk tunneling only results in minor injury to the plant.
Scouting method: A scouting method has not been developed for the black sunflower stem weevil.
Economic threshold: None has been established.
Management: Insecticide use has generally not been justified for control of the black sunflower stem weevil.
Thistle Caterpillar (Painted Lady Butterfly)
Species: Vanessa cardui (Linnaeus)
Description: The adult butterfly is about 1 inch long with a wingspread of about 2 inches (Figure 55). The upper wing surfaces are brown with red and orange mottling and white and black spots. The undersides of the wings are marble gray, buff and white. Each hind wing possesses a row of four distinct and obscure eyespots. Eggs are small, spherical and white. The larvae are brown to black and spiny, with a pale yellow stripe on each side (Figure 56). When mature, the larvae are 1.25 to 1.5 inches long. The chrysalis, or pupa, is molten gold and about 1 inch long.
.jpg)
Life cycle: The painted lady butterfly is indigenous to the southern U.S. and migrates annually to the northern U.S. and Canada. The painted lady breeds in the north-central states and Canada, migrates south for the winter and returns to the northern areas in early June. Eggs are laid on Canada thistle, wild and cultivated sunflower, soybean and many other host plants. Hatching occurs in about one week. Larvae are called thistle caterpillars and feed on sunflowers until they reach maturity in late June or early July. Chrysalids are formed and hang from the leaves of the plant. Butterflies will emerge in about 10 days from the chrysalid and a second generation begins.
Damage: Thistle caterpillars (larvae) feed on the leaves and, when numerous, may defoliate infested plants. Larvae produce a loose silk webbing that covers them during their feeding activity. Black fecal pellets produced by the larvae often are found in proximity to the webbing.
The effect of defoliation by the larvae on the yield of sunflower is similar to that described for defoliation by sunflower beetle larvae.
Scouting method: Sampling sites should be at least 75 to 100 feet from the field margins when collecting data to determine whether an entire field should be treated. Infestations frequently will be concentrated in areas of a field where Canada thistle plants are abundant. Plants should be examined carefully for the presence of eggs and/or larvae.
The field should be monitored by using the X pattern, counting 20 plants per sampling site for a total of 100 plants to determine percent defoliation (Figure 38).
Economic threshold: The threshold is 25% defoliation, provided that most of the larvae are still less than 1.25 inches long. If the majority of the larvae are 1.25 to 1.5 inches long, most of the feeding damage already will have occurred and treatment is not advised.
Management: Insecticide use generally has not been justified for control of larvae of the painted lady. However, instances of high localized infestations have occurred within certain fields where spot treating may be necessary. When large populations of caterpillars are present in fields, disease epidemics can occur as indicated by dying larvae on leaves (Figure 57).
.jpg)
Sunflower Midge
Species: Contarinia schulzi Gagné
Description: The tan body of the adult sunflower midge is about 0.07 inch long, with a wingspan of about 0.2 inch (Figure 58). The wings are transparent with no markings except the veins. The larvae attain a length of nearly 0.1 inch at maturity and they are cream to yellowish orange when fully grown (Figure 59). Midge are tapered at the front and rear, with no legs or apparent head capsule.

- Figure 58. Adult – Sunflower midge. (Extension Entomology)
.jpg)
Life cycle: The sunflower midge overwinters in the soil as a cocooned larva and pupates during June and July in North Dakota and Minnesota. Typically, the initial peak of first-generation adult emergence occurs in early to mid-July. A second peak occurs about seven to 10 days later. They prefer to lay eggs on sunflower buds with a diameter greater than 1 inch. Larvae initially feed on margins of the head between the bracts surrounding the heads. Larvae migrate to the base of the developing seeds and to the center of the head as it develops. Presence of the larvae frequently is determined by necrotic areas at the base of or between the bracts. As midge larvae mature, they move to the surface of the head and drop to the ground. A partial, second generation occurs in August. Second-generation adults oviposit among the seeds.
Damage: Damage to sunflower is a result of first-generation larval feeding in developing heads. When populations are low, damage is restricted to the base of the bracts of the head and causes slight localized necrosis but little if any economic loss. When many larvae are present, feeding prevents ray petal formation and distorts the growth of the developing sunflower head. If the abnormal growth is severe, the back of the head overgrows the front and little or no seed production occurs (Figure 60). If an infestation occurs in the early bud stage, the bud may be killed.

- Figure 60. Severe damage to receptacle and seed development occurs when midge infection is high.(Extension Entomology)
.jpg)
Often midge damage is restricted to field margins or small portions of fields and economic losses are minimal. However, when populations are very heavy, damage will extend throughout the field and substantial economic losses occur. The extent of damage from second generation larvae is unknown.
Scouting method: None has been established.
Economic threshold: None has been established.
Management: Because effective chemical and other controls are not available, sunflower midge management relies on cultural practices done prior to planting. If a midge infestation is anticipated, new fields should be established away from fields damaged the previous season. To minimize the risk of all plantings being at their most susceptible stage at midge emergence, several planting dates should be used. Late planting dates reduce midge infestations because adult emergence and egg laying are usually completed before the susceptible crop stage. If available, growers should consider using a tolerant hybrid.
Red Sunflower Seed Weevil
Species: Smicronyx fulvus LeConte
Description: Red sunflower seed weevil adults are 0.1 to 0.12 inch long and reddish brown (Figure 61). The larvae are small, 0.1 inch long, cream-colored, legless and C-shaped (Figure 62).
.jpg)
Life cycle: Red sunflower seed weevil emergence occurs in late June and early July. The newly emerged adults feed on sunflower buds or floral tissues. Once pollen is available, the adults include it in their diet. Females need to feed on sunflower pollen for several days prior to egg deposition. Eggs are deposited within young developing seeds. Normally a single egg is placed in each seed, although 8% to 12% of the seeds may contain several eggs.
Small, white eggs hatch in approximately one week. Larvae consume a portion of the kernel, and this feeding causes economic damage. After completion of larval development, the majority of the larvae drop to the ground. Larval drop occurs from mid-August through September. The larvae overwinter in the soil at a depth of about 6 inches. Larvae pupate in late June of the following year and the pupal period lasts about one week. A single generation per year is produced in the northern Great Plains.
Damage: While the kernel of some seeds may be totally eaten, most seeds are only partially consumed. The separation of undamaged from weevil-damaged seed is difficult.
Most larvae drop from the head to the soil after completing their development, but a small percentage may remain in the seed and are present at harvest. Growers who encounter a seed weevil infestation may want to delay harvest to allow most of the weevil larvae to exit the seeds to avoid having larvae in the harvest bin.
Larvae that are still in the seed at bin filling time are done feeding and can cause heating and moisture problems. Larvae harvested with the seed cannot be controlled until they have completed development and have emerged from the infested seeds. Once emerged, they are susceptible to fumigation. However, fumigation normally is not recommended. The most advantageous time to initiate control of seed weevil is in the field when the adult weevils are active, but prior to egg deposition.
Economic thresholds: The economic threshold varies with differences in plant population, the cost of insecticide application and the market price of sunflower. The procedure for calculating the economic threshold is discussed in the NDSU Extension sunflower seed weevil publication (E817 revised). Currently, an infestation level of four to six seed weevil adults per head in oil sunflower or one seed weevil per head in confectionery sunflower is the average economic threshold.
The optimal period for insecticide treatment is when at least three out of 10 plants in the field are at early bloom (R5.1 to R5.4, Figure 2) and the economic threshold has been reached. If spray application is delayed past when more than four out of 10 plants are at stage R5.4, many eggs already will be laid in the developing seeds and those eggs and larvae cannot be controlled. If fields are sprayed too early, reinfestation may occur in areas with a high weevil population. After spraying, fields should be rechecked periodically to determine if reinfestation is reaching the economic threshold. Continue rechecking until most of the heads in the field have reached the R5.7 stage. At that stage, most eggs already will have been laid and most seeds will be too mature to be suitable for further red seed weevil egg oviposition.
Scouting method: Begin by taking samples from 12 plants, three plants from each of the four field sides. Sampling sites should be at least 75 feet in from field borders, which often have an inordinately high number of weevils. The total number of weevils counted should be compared to the sequential sampling table in the most recent NDSU Extension sunflower seed weevil publication (E817 revised). According to the table, take one of three possible actions: Stop sampling, no action is needed; stop sampling and treat; or take more samples because a decision cannot be reached. When populations are low or high, sequential sampling allows a quick decision with few samples. If populations are near the economic threshold, more precision is needed for making an accurate determination and more samples are required.
Note: To more precisely check individual sunflower heads for red sunflower seed weevils, the face of the heads should be sprayed with a commercial formulation of mosquito repellent containing diethyl toluamide (DEET). This will cause the weevils to move out from between the florets and they can be more accurately counted. Consult the most recent NDSU Extension sunflower seed weevil publication (E817 revised) for a table to convert the visual counts to the absolute number of weevils (counted and uncounted).
Management: Insecticides are commonly used for control of the sunflower seed weevils.
Early planting of sunflower reduces seed damage caused by the red sunflower seed weevil without causing a measurable reduction in oil content and seed weight.
Surrounding a sunflower field with a ring of early blooming sunflowers effectively can trap immigrating red sunflower seed weevils into a small portion of the field, where they can be controlled efficiently. The trap cropping method given in publication E817 (revised) is as effective and more cost efficient than standard insecticide treatment for control of red sunflower seed weevils. However, trap cropping has not been widely adopted by sunflower growers.
Sunflower hybrids differ in their susceptibility to the red sunflower seed weevil. A line with resistance to red sunflower seed weevil, HA 488, was released by the USDA-Agricultural Research Service, Fargo, N.D., in 2020 as a germplasm source for host plant resistance breeding.
For more information, see the NDSU Extension publication E817 (revised), “Integrated Pest Management of Sunflower Seed Weevil.”
Gray Sunflower Seed Weevil
Species: Smicronyx sordidus LeConte
Description: Adults of the gray sunflower seed weevil are slightly larger (0.14 inch long) than S. fulvus and gray (Figure 63). The larvae are small, 0.12 inch long, cream-colored, legless and C-shaped (Figure 64).
.jpg)
.jpg)
Life cycle: Gray sunflower seed weevil emergence occurs in late June and early July and reaches 50% emergence about 10 days before the red sunflower seed weevil. The newly emerged adults feed on floral buds. Oviposition occurs on flowers in the bud stage and before red sunflower seed weevil oviposition begins. Female gray sunflower seed weevils do not lay as many eggs as do females of the red sunflower seed weevil.
The larvae feed in a single seed, and infested seeds are enlarged and protrude above surrounding uninfested seeds. The majority of the larvae drops to the ground from mid-August through September and overwinters in the soil. Larvae pupate in late June. A single generation per year is produced.
Damage: Seeds infested by the gray seed weevil lack a kernel and, due to their light weight, the seeds may be lost during the harvesting process. Because of their low population levels and low fecundity, the gray sunflower seed weevil usually does not cause economic damage, especially in oil sunflower fields. In confection fields, however, populations of the gray sunflower seed weevil may be sufficiently high to warrant treatment at the late bud stage (R3 to R4).
As with the red sunflower seed weevil, larvae normally drop from the head to the soil after completing their development. Larvae that do not emerge will present the grower with the same bin problem as unemerged red sunflower seed weevil larvae.
Scouting method: Normally, gray sunflower seed weevil populations are too low to cause economic damage. However, if an area has had a history of high populations, fields, especially confection fields, should be sampled beginning at bud stage R2 (Figure 2). Sampling should be conducted as for the red sunflower seed weevil and continue until plants are blooming.
Economic thresholds: None has been established.
Management: If fields are to be treated with insecticides, they need to be sprayed while the plants are still in the early bud stage. By late bud stage, most oviposition already will have occurred.
Sunflower Moth
Species: Homoeosoma electellum (Hulst)
Description: The adult is a gray to grayish tan moth about 0.38 inch long, with a wingspan of about 0.75 inch (Figure 65). The hind wings are devoid of markings; however, the forewings have a small, dark, discal dot near the center of each wing and two or three small, dark dots near the leading margin of each wing. When at rest, the wings are held tightly to the body, giving the moth a somewhat cigar-shaped appearance. The larva has alternate dark and light-colored longitudinal stripes on a light brown body (Figure 66). The larva is about 0.75 inch long at maturity.

Life cycle: Sunflower moth migrations from the south-central U.S. normally appear in North Dakota in early to mid-July. The moths are highly attracted to sunflowers that are beginning to bloom. Individual female moths will deposit up to 30 eggs per day on the surface of open sunflower heads. Eggs hatch within 48 to 72 hours and the newly emerged larvae feed on pollen and florets. Larvae begin tunneling into seeds upon reaching the third instar (larval growth stage). This tunneling continues throughout the remainder of larval development. Larval development from hatching to full maturity takes about 15 to 19 days.
Damage: The young larvae of the sunflower moth feed primarily on florets and pollen. Older larvae tunnel through immature seeds and other parts of the head. A single larva may feed on three to 12 seeds and forms tunnels in the seeds and head tissue. Larvae spin silken threads, which bind with dying florets and frass to give the head a trashy appearance. Severe larval infestations can cause 30% to 60% loss, and in some cases, the entire head can be destroyed. Sunflowers infested with sunflower moth have an increased incidence or risk of Rhizopus head rot.
Scouting method: Sampling sites should be at least 75 to 100 feet from field margins. The X pattern should be used in monitoring a field. Count moths on 20 heads per sampling site for a total of 100 heads. Scouting is most accurate in the early morning or late evening, when moths are active. Sex pheromone lures are available commercially for monitoring with traps to indicate their arrival and local populations.
Economic threshold: The economic threshold for sunflower moth is one to two adults per five plants at the onset of bloom or within seven days of the adult moth’s first appearance. If using pheromone traps, insecticide applications should be considered when an average of four moths per trap per day are captured from the R3 through R5 growth stages.
Management: Insecticides are commonly used for control of the sunflower moth.
Banded Sunflower Moth and Arthuri Sunflower Moth
Species: Cochylichroa hospes Walsingham and Cochylichroa arthuri Dang
Description: The adult has a dark band across the buff or yellowish-tan forewings (Figure 67). The wingspan is about 0.5 inch. A related species, Cochylichroa arthuri Dang, also infests sunflowers in the northern Great Plains. Arthuri sunflower moth has comparable feeding habits and development, and causes damage similar to that of the banded sunflower moth. Distinguishing Arthuri sunflower moth from the banded sunflower moth is not necessary for pest management purposes. Arthuri sunflower moth is a small, whitish-gray moth with a wingspan of about 0.5 inch. Its forewings are crossed by a broken brown and gray band and the outer ¼ has brownish markings and dark fringe (Figure 68). Early instar larvae are off-white; late instar larvae are pinkish to red with a brown head capsule (Figure 69). Larvae will be about 0.44 inch at maturity.
.jpg)

- Figure 68. Adult – Arthuri sunflower moth. (Gerald Fauske, NDSU)
.jpg)
Life cycle: The life cycle of the banded sunflower moth is similar to that of the sunflower moth, except that the adults emerge from local overwintering sites rather than migrating into North Dakota. Banded sunflower moths begin to emerge from the soil about mid-July and are present in the field until mid-August. Adults tend to congregate in field margins on weeds or adjacent crops during the day and then move into the crop in the evening. Within a week after emergence, they begin to lay eggs on the outside of the bracts of the sunflower head. Eggs may be found through early August and hatch in five to eight days. Larvae develop through five instars and are present in sunflower heads from mid-July to mid-September. After feeding to maturity, larvae drop to the ground and spin cocoons in the soil to overwinter. Pupation takes place in late June or early July the following year. The pupal period lasts about 12 days.
Damage: The newly hatched larvae move from the bracts to the florets of the sunflower head, where they enter open florets to feed. When the eggs hatch, young larvae feed on bract tissue before moving into the head. A sunflower head is susceptible to infestation only during the flowering period. The larvae feed in the florets until the third instar. During later stages of larval development, the insect tunnels through the base of the floret into the seed. The larvae may consume part or all of the contents of the developing seed. The larvae usually enter near the top of the seed and leave by way of the same opening after the contents are eaten. Each larva may destroy five to seven seeds. Small areas of silken webbing on mature sunflower heads indicate the presence of banded sunflower moth larvae within the head.
Adult scouting method and economic threshold: Sampling sites should be at least 75 to 100 feet from the field margins. In monitoring a field, use the X pattern (Figure 17), counting moths on 20 plants per sampling site to obtain the total number of moths per 100 plants. Sampling should be conducted during early flowering, usually during mid-July. If treatment is justified, it should be applied at the R5.1 sunflower plant growth stage (when 10% of head area has disk flowers that are flowering or completed flowering.)
During the day (late morning to early afternoon) the moths remain quiet, resting on upper or lower surfaces of the leaves of sunflower plants. When disturbed, they flutter from plant to plant. When sampling for moths during the day, the decision to treat or not is based on comparing the mean number of adult moths in the field to the EIL for moths. The EIL number is the number of moths per head that will, if not managed, result in seed damage with a value equal to the cost of treatment. Use the following formula based on treatment costs, plant population and market price to determine the adult EIL for day sampling:
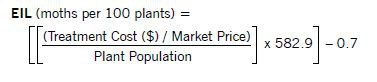
The constants in the formula simplify the calculation and include the amount of loss attributable to each banded sunflower moth larva produced per plant.
A sample calculation of the EIL based on moth sampling for the following conditions is given below.
Insecticide treatment cost = $8/acre
Market price = $0.18/lb.
Plant population = 20,000/acre
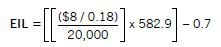
= 0.595 which for observational purposes would equal
1 moth per 100 plants
For this set of variables, an infestation of about one moth per 100 plants will result in sufficient larvae to destroy seeds in the sunflower head equal to the $8 treatment cost per acre in a field of 20,000 plants per acre with a market value of 18 cents per pound. If the adult population has reached or exceeded this level, then the grower should consider the use of a chemical insecticide to prevent larval seed damage.
Egg scouting method and economic threshold: Banded sunflower moth eggs can be counted accurately using a low power 3.5X head-mounted magnifier. Egg counts should be made when most of the sunflower plants are at the late bud stage R3. However, buds should be selected randomly to avoid bias. Sampling for banded sunflower moth egg populations in commercial fields should be conducted as follows:
- Divide each side of the field to be surveyed into 1,312-foot sections.
- Sample the center of each 1,312-foot section at 20 feet into the field from the field margin.
- Randomly select five buds at each sample site.
- Randomly select six bracts from the outer whorl on each bud and count the banded moth eggs. Average the egg counts from the five buds.
Compare the average egg count to the calculated EIL (below).
Economic injury level (EIL) is the number of eggs per six bracts.
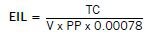
V = Market value per lb
PP = Plant population per acre
TC = Treatment cost
Example: TC = $8, V = $0.18 and PP = 20,000
The EIL is 2.9 eggs per six bracts.
Management: Insecticides are commonly used for control of the banded sunflower moth.
Research in North Dakota has demonstrated that delaying planting of sunflower until late May or early June helps reduce infestation levels of the banded sunflower moth.
Sunflower hybrids differ in their susceptibility to banded sunflower moth. A line with resistance to banded sunflower moth, HA 489, was released by the USDA-ARS, Fargo, N.D., in 2019 as a germplasm source for host plant resistance breeding.
For more information, see the NDSU Extension publication E823 (revised), “Banded Sunflower Moth.”
Lygus Bugs
Species: Tarnished plant bug, Lygus lineolaris (Palisot de Beauvois) and other Lygus species
Description: The most common species occurring in sunflower fields is the tarnished plant bug. It attacks at least 385 different plant species and occurs in 39 U.S. states and five Canadian provinces. Adults (Figure 70) are small, cryptically colored insects with a distinctive yellow triangle or “V” on the wings and 0.2 inch in length. They vary from pale green to dark brown. The immature stages, or nymphs (Figure 71), are similar in appearance to the adults but lack wings and are usually green. They often are confused with aphids, but Lygus move much more rapidly and lack cornicles on the posterior abdomen.

- Figure 70. Adult – Lygus bug (tarnished plant bug).(Scott Bauer, USDA, Bugwood.org)

- Figure 71.Nymph – Lygus bug (tarnished plant bug).(Scott Bauer, USDA, Bugwood.org)
.jpg)
Life cycle: Adults overwinter in plant debris along field margins and shelterbelts. Populations probably move to sunflower from alfalfa, canola or other crops when those plants have senesced or been harvested. Sticky trap catches in North Dakota showed that Lygus bugs were present throughout the reproductive growth stages of sunflowers. These insects produce at least two generations per year in the northern Plains. The biology of other Lygus species is similar.
Damage: Oilseed sunflower are usually not at risk for feeding injury from the Lygus bug. The presence of scarring on confection sunflower seeds, known as kernel brown spot (Figure 72), is caused by Lygus bugs feeding on the developing seed. The quality issue is significant because processors discount the finished product with only 0.5% damage. Lygus feed preferentially on the developing reproductive organs or on the apical meristematic and leaf primordial tissue, causing a necrosis around the feeding site due to the injection of enzymes. This tissue destruction causes the brown spot on the sunflower kernel, resulting in a bitter taste to the seeds. Greenhouse and field studies showed that 33 to 38 seeds were damaged per adult Lygus bug, and that all reproductive growth stages (R4 to R5) were vulnerable to attack. Damage was reduced if heads were infested after flowering was completed (R6 to R7).
.jpg)
Scouting method: A scouting method has not been developed for Lygus bug in sunflowers.
Economic threshold: Approximately 36 seeds are damaged by each adult. Therefore, 0.5% damage on heads with 800 seeds would occur with feeding on only four seeds per head. Thus, populations of adult Lygus at levels of one per nine heads could result in economic loss to the producer through the reduction of seed quality.
Management: Lygus can be treated at the same time confection sunflower is treated for other insects, such as the seed weevil and banded sunflower moth. Two treatments are recommended to sufficiently protect confection sunflower heads from insect feeding: one application at the onset of pollen shed, or approximately 10% bloom, followed by a second treatment seven days later. This program should control insects adequately on confection sunflower throughout flowering, minimizing the potential feeding damage.
Diseases of Sunflower
Sam Markell, Febina Mathew and Bob Harveson
Sunflowers growers will see a variety of diseases every year. In many cases, these diseases can be economically important if the environmental conditions are favorable for infection and spread.
Management of diseases in sunflower should be a priority of sunflower growers in North Dakota, and accurate identification of diseases is a critical first step toward disease management. In this chapter, we describe the frequency, biology, signs and symptoms, and management tools for 17 diseases in sunflower that growers are likely to see in North Dakota and other northern Great Plains states. The chapter is organized into four sections: Foliar Diseases, Stem/Wilt Diseases, Head Diseases and Other Diseases.
Foliar Diseases
Alternaria Leaf Spot
(Alternariaster helianthi, species of Alternaria)
Alternaria leaf and stem spot is a disease commonly found in the northern Great Plains but is not generally considered to be economically important in this region. The causal fungus survives on infested residue, and optimal conditions for infection and disease development are temperatures of 77 to 82 F and frequent periods of leaf wetness exceeding 12 hours.
The disease is most commonly found toward the end of the season on older (and often senescing) leaves in the lower canopy. Leaf lesions begin as small, irregular dark spots, which may be surrounded by a lighter colored halo (Figure 73). Lesions may enlarge, coalesce and cause the leaf to wither.
Stem lesions begin as randomly distributed (i.e., not associated with a petiole, etc.) dark colored flecks that enlarge to narrow elliptical or linear lesions about ½ to 1½ inches long. Lesions can occur on the back of the head, appearing as dark spots that may be sunken.
Active management specific to Alternaria leaf spot in the northern Great Plains is not needed. However, the disease causes severe yield losses in warmer and more humid climates, and yield losses have been reported when sunflower is grown in the southeastern U.S. and other sunflower-producing countries. Crop rotation, residue management and use of foliar fungicides may be recommended in regions where the disease is a problem.
.jpg)
Bacterial Leaf Spot
(Pseudomonas syringae pv. helianthi)
Bacterial leaf spot is not an economically important disease in the northern Great Plains. The pathogen can be seed-, soil- or residue-borne. Optimal conditions for bacterial infection and disease development include wounding of leaf tissue (hail, sandblasting, high winds, etc.) associated with warm (75 to 90 F) and humid (relative humidity greater than 90%) conditions.
Lesions appear as irregular to angular-shaped brown to black spots of varying size on leaves, petioles and stalks that may be surrounded by a yellow halo (Figure 74). Leaf spots will enlarge, often forming linear lesions that dry, crack and eventually fall out. Infections typically are restricted to the most humid part of the canopy (lower leaves). Active management specific to bacterial leaf spot is not needed in the northern Great Plains.

Powdery Mildew
[Golovinomyces cichoracearum (syn. Erysiphe cichoracearum), Golovinomyces ambrosiae, Podosphaera xanthii]
Powdery mildew is a very common disease but is seldom economically important in the northern Great Plains. Disease occurrence is generally very limited until bloom, but by plant maturity, powdery mildew is readily found in the majority of fields in this region.
The fungi causing powdery mildew disease are very host specific; thus, the fungus infecting sunflower does not spread to other crops, and vice versa. The fungus survives in resting structures (chasmothecia) or as mycelium on crop residues.
Optimal conditions for infection include high humidity and moderate temperatures (68 to 81 F). Infection begins (and disease severity is the greatest) on leaves in the lower canopy, where conditions are most favorable.
Powdery mildew often is observed first as small, discrete, powdery white tufts of fungal growth on the top side of leaf tissue (Figure 75). Under favorable conditions, the fungal growth quickly can cover an entire leaf, and also may occur on bracts or the back of the head (Figure 76). Small pepper flake-sized black structures (chasmothecia) may be produced within the white fungal growth in severely infected leaves.
Importantly, the fungal growth only occurs on the upper side of the older leaves, distinguishing the disease from downy mildew, where the white fungal growth is only observed on the underside of the leaf tissue. Active management specific to powdery mildew is generally not needed in our region; however, crop rotation, selection of a less susceptible sunflower hybrid and foliar fungicide application may be recommended in other production regions.

- Figure 75. Discrete white fungal tufts of powdery mildew on the underside of the leaf.(Sam Markell, NDSU)
.png)

- Figure 76. Powdery mildew on top surface of the leaf.(Sam Markell, NDSU)
.jpg)
Rust
(Puccinia helianthi)
Rust is the most economically important foliar disease of sunflower in the U.S. The disease can be found every year in the northern Great Plains, but severity varies widely.
Oilseed hybrids tend to be less susceptible than nonoilseed hybrids. In extreme cases, yield losses exceeding 80% have been reported in North Dakota. Active scouting and management of rust is strongly recommended.
The pathogens that cause rust diseases are very host specific; thus, sunflower rust, wheat leaf rust, dry edible bean rust and other rust diseases commonly occurring in North Dakota cannot spread between crops. The sunflower rust fungus survives even the harshest winter because spores can survive on wild, volunteer or commercial sunflower residue.
Favorable conditions for rust infection include frequent periods of leaf wetness (fog, dew) and temperatures between 55 and 85 F. A rust epidemic may begin in sunflower fields anytime in the growing season.
An early epidemic of rust begins when the pathogen overwinters and completes its life cycle in (or in proximity to) a sunflower field. When this occurs, yellow to orange raised bumps (pycnia) about c to ¼ inch in diameter appear on the top side of leaves or cotyledons, and a similarly sized cluster of orange cups (aecia) appear opposite them on the underside of the leaf or cotyledons (Figures 77-78).
Small raised cinnamon-brown pustules (uredinia) filled with dusty brown urediniospores typically occur within a week or two of aecia observation on leaf tissue (Figure 79). Pustules may be surrounded by distinct chlorotic halos. More commonly, rust appears in the late vegetative or early/mid reproductive growth stages, when sunflowers are infected by uredinisopores that are aerially dispersed from a more distance source (such as infected commercial or wild sunflowers several miles away).
Under favorable conditions, urediniospores can cause new infections to produce new uredinia and urediniospores every seven to 14 days. When rust is severe, pustules also can be found on stems, petioles and bracts.
The severity of a rust disease epidemic is linked to the number of urediniospore infection cycles in the season; thus, yield loss can be very severe when an epidemic begins in early vegetative growth stages and frequent heavy dews of fog occur during the growing season. At the end of the growing season, the pustules transition into their overwintering stage (telia) and turn hard and black (Figures 80-81).
Rust is best managed using multiple tools and techniques, including destruction of overwinter hosts (volunteers, wild sunflowers), avoiding planting next to a field with infested residue, selection of a sunflower hybrid with genetic rust resistance and application of an efficacious foliar fungicide. Scouting sunflower fields for rust is critical when considering a foliar fungicide application because not every field gets rust every year and the timing of onset and severity varies.
The action threshold for application is 1% rust severity on the upper four fully expanded sunflower leaves at or before bloom (growth stage R5) (Figure 82). However, when a nonoilseed hybrid is infected from overwintering infection, an earlier application may be justified.
.jpg)
.jpg)

- Figure 79. Rust uredinia. (Andrew Friskop, NDSU)
.jpg)
.jpg)
.jpg)
Septoria Leaf Spot and Blight
(Septoria helianthi)
Septoria leaf spot and blight is a common disease in the northern Great Plains but is not considered economically important in the northern sunflower growing region. The pathogen can be residue- or seed-borne, and is favored by frequent rains.
Lesions first appear on the lower leaves as very small yellow to dark brown spots (pinhead size). These spots enlarge to circular lesions (up to ¾ inch in diameter) that scatter mostly on the upper side of the leaf blade (Figure 83). The lesions may be delimited by veins in the leaves, which gives them an angular or diamond shape.
Through time, the lesions coalesce and the fungus gradually spreads from the lower leaves to upper leaves. The lesions also may contain very small fungal reproductive structures (pycnidia), which appear as black specs or bumps when using a hand lens (Figure 84).
This disease most frequently occurs toward the end of the growing season, although visible lesions early in the growing season are not uncommon. The disease often goes dormant in periods of warm and dry weather.
Active disease management specific to Septoria leaf spot is not needed in the northern sunflower growing region; however, clean seed, tillage and crop rotation typically are recommended for disease management. Foliar fungicides are labelled to control Septoria leaf spot but would not be recommended unless the disease is found to be economically important in the northern sunflower growing region.

- Figure 84. Septoria leaf spot. (Charlie Block, Iowa State University)
.jpg)
.jpg)
Stem/Wilt Diseases
Bacterial Stalk Rot (Pectobacterium carotovorum subsp. carotovorum, P. atrosepticum)
Bacterial stalk rot occurs only sporadically and is not an economically important disease in the northern Great Plains. The causal pathogen is ubiquitous and is spread by splashing and wind-driven rain. The bacteria can enter the sunflower stem only through a wound; thus, plant damage caused by hail, sand blasting, birds, insects or mechanical injury is a prerequisite to infection.
Externally, infected stems feel “softened” and may appear dry or brown. Internally, stems become blackened and slimy, and ooze or foam may appear as a result of bacteria digesting plant tissues. Stems may lacerate or explode under the pressure of fermentation (Figures 85-86).
A robust and putrid smell, resembling that of rotten potatoes, is often the first noticeable symptom of bacterial stalk rot. Notably, the same bacteria cause bacterial head rot of sunflower, and disease symptoms are similar. Active management of bacterial stalk rot is not needed.

- Figure 86.Bacterial stalk rot.(Bob Harveson,University of Nebraska)
.jpg)
.jpg)
(Macrophomina phaseolina)
Charcoal rot is capable of causing yield loss in hot and dry environments. The disease is most prevalent and of economic concern in the southern Great Plains and central high Plains sunflower production regions, and is important in hot, dry growing seasons in the northern Great Plains.
The fungus is soil-borne, survives as microsclerotia and can infect several hundred weeds and crops, including corn and soybean. The disease is favored by very high temperatures (greater than 90 F) and low moisture in the soil during the reproductive growth stages of sunflower development.
Infection can occur through the roots early in the growing season but commonly only manifests in late reproductive growth stages (after flowering) and under moisture stress. External symptoms and signs include a silvery or charcoal colored basal stem lesion at the soil line, and the loss of stem integrity, which can lead to premature senescence or lodging (Figure 87). Internally, stems may be filled with dusty black microsclerotia, causing tissues to appear as if dipped in charcoal dust (Figure 88).
Microsclerotia appear as circular specks under a magnification by a hand lens (Figure 89). Management tools include crop rotation with nonhosts (for example, wheat), using a relatively lower plant population, practicing conservation tillage and by production practices mitigating drought stress.

- Figure 89. Charcoal rot inside the stem. (Sam Markell, NDSU)
.jpg)
.jpg)
Downy Mildew
(Plasmopara halstedii)
Downy mildew is common and economically important in the northern Great Plains, particularly in North Dakota and northern Minnesota. When infection occurs in sunflower seedlings, the disease is systemic and eventually will result in 100% yield loss of every infected plant. However, total yield loss in a field depends on the number of plants infected and their distribution.
The pathogen is host specific and soil-borne, and can survive many years in the soil. The disease is favored by cool and wet/waterlogged soil conditions when the seeds are germinating and seedlings are emerging. Infection begins when motile zoospores, which swim in water, infect the radicles and roots of germinating seed and seedlings. Infected seedlings may die pre- or postemergence.
Surviving plants display systemic chlorosis on the upper side of emerging leaves, which appears to radiate outward from the petioles (Figures 90 and 91). During periods of humid weather, fluffy white growth will appear only on the underside of the leaves, opposite the chlorosis (Figure 92). This is diagnostic and distinguishes the disease from powdery mildew, herbicide damage and other abiotic or biotic ailments.
If plants survive through the season, they remain extremely stunted (often 6 to 24 inches tall) and form a horizontal head with limited and nonviable seed (Figure 93). Downy mildew is frequently concentrated in low/wet spots of the field, but sporadically infected plants throughout the field are not uncommon. Areas in the field with a high concentration of infected sunflower plants often appear as if they were not (or minimally) seeded and quickly fill with weeds.
Nonsystemic downy mildew infection can occur later in the growing season and appears as discrete irregular chlorotic spots on the upper side of leaves and occasionally white growth opposite them. However, this nonsystemic infection is not economically important (Figure 94).
Selection of hybrids with genetic resistance and application of a fungicide seed treatment are the only viable management tools. However, the pathogen is highly genetically variable and has adapted to (and overcome) multiple resistance genes and fungicide mode of actions. Thus, consulting the most current recommendations before selecting resistant hybrids or fungicide seed treatments is critical.
.jpg)

- Figure 94. Downy mildew as a secondary infection. (Sam Markell, NDSU)
.jpg)
Fusarium Root and Stem Rot
(species of Fusarium)
How frequently Fusarium root and stem rot occurs in the northern Great Plains is unclear. Similarly, while economic damage can occur, it is thought to be limited. Multiple species of Fusarium are known to cause disease in sunflower, and conditions favoring infection are wet weather during midseason followed by warm/hot temperatures (80 to 100 F) and dry weather.
Fusarium stem rot frequently is associated with charcoal rot, complicating the current understanding of its potential economic importance. Species of Fusarium causing disease on sunflower are soil- or residue-borne and infect plants through the roots. Severely infected plants can be noticed by premature senescence and exterior browning of the stem (Figure 95). When the stems of infected plants are cut lengthwise, pinkish to brown discoloration of the inside tissue is observed (Figure 96).
Active management specific to Fusarium root and stem rot is not needed, given the disease is of limited economic importance. However, the disease may be mitigated by reducing plant stress.
.jpg)
Phoma Black Stem
(Phoma macdonaldii)
Phoma black stem is the most commonly occurring stem disease in the northern Great Plains. Yield loss has been documented in North Dakota, but it is thought to be rare.
The fungus survives on infested residue. Infection occurs when fungal spores are splash dispersed from residue and are deposited on leaves during a period of free moisture. Infection often begins on leaf margins, and a chlorotic and necrotic angular lesion will form and progress across the leaf, through the petiole and into the stem.
A small (approximately 2 inches long by 1 inch wide) coal-black stem lesion forms on the stem at the base of the petiole (Figures 97-99). The lesion will remain superficial and healthy white tissue is apparent if the lesion is shaved off. Many lesions can occur on the same plant.
Because rain splash and high humidity are needed to begin the disease cycle, infected leaves (and subsequent stem lesions) often are limited to the lower half of the plant. Yield loss may occur if the pathogen is vectored inside and within the stem by burrowing sunflower stem weevils (Apion) or in portions of fields with very high yield potential.
Crop rotation, avoiding planting adjacent to previously cropped sunflower residue and selection of a less susceptible hybrid (if known) may help manage the disease. Foliar application of an efficacious fungicide also can manage the disease effectively, but it is thought to be rarely economically viable.
.jpg)

- Figure 98. Phoma black stem. (Sam Markell, NDSU)

- Figure 99. Phoma black stem (black on the right) and developing Phomopsis stem canker lesion (brown). (Sam Markell, NDSU)
.jpg)
.jpg)
Phomopsis Stem Canker
(Diaporthe helianthi, D. gulyae, species of Diaporthe)
In the 2010s, Phomopsis stem canker emerged as the most economically important stem disease in the northern Great Plains. Prevalence of the disease has varied among years and locations, and is generally highest in seasons with frequent rain events. In severely infected fields, shriveled heads and/or lodged plants can result in near total yield loss.
The causal fungus overwinters on infested sunflowers or alternative host residue. Infection begins when fungal spores are splash dispersed from residue to leaves, which experience periods of free moisture. Infections often begin at leaf margins, forming large triangular bronzed to brown lesions that quickly spread into the petiole and stem.
Brown stem lesions originate at the petiole, spread up and down the stem (and may girdle the stem), expanding to a length often greater than 6 inches (Figure 100). The stem weakens and “hollows out” under the lesions, where it easily can be punctured by light thumb pressure. Infected plants wilt, lodge and/or die (Figure 101).
Even when using every management tool available, Phomopsis stem canker may not be adequately controlled in favorable environments for disease development. Crop rotation, elimination of residue, diligent control of volunteer and wild sunflowers, and strategically avoiding planting near a previously infected crop are important. A four-year crop rotation with nonhosts (for example, wheat and corn) may lower the pathogen survival, but research data is lacking.
Selection of a less susceptible (or partially resistant) hybrid is critical in areas prone to Phomopsis stem canker. Foliar application of an efficacious fungicide at early reproductive growth stages (R1 growth stage) may help mitigate the disease, but only will provide partial control of the disease. Research optimizing fungicide timing and selection is ongoing, and consultation of the most recent recommendations is justified before making an application.
.jpg)
Verticillium Wilt
(Verticillium dahliae, V. albo-atrum)
Verticillium wilt is found sporadically in the northern Great Plains but is economically damaging when it occurs. The fungus is soil-borne, survives as microsclerotia and has a wide host range, which includes potato and several broadleaf weeds.
Infection begins when germinating microsclerotia infect growing sunflower roots. The disease progresses inside the stem, initially producing a brown ring in the vascular tissue (Figure 102). The outside of the pith will be colonized in gray-black powdery microsclerotia, eventually detaching from stem tissue, leaving a hollowed stem with limited integrity (Figure 103).
In severely infected plants, the exterior of the stem may be coated with dusty silver-black microsclerotia that can be scraped off easily with a fingernail. Although a root/stem disease, symptoms are first noticed in leaves. Interveinal chlorosis (yellowing) and necrosis (browning) occur earliest (and most severely) on the lower leaves and appear to progress upward on the plant (Figures 104-105).
Verticillium wilt commonly occurs in small clusters of sunflower plants, and yield loss occurs as plants wilt and die in reproductive growth stages. Crop rotation with nonhosts (for example, wheat and corn), avoiding fields with a history of the disease and selection of a resistant sunflower hybrid (if available) are important management tools for Verticillium wilt.


- Figure 105. Verticillium wilt leaf symptoms. (Sam Markell, NDSU)
.jpg)
Sclerotinia Wilt/Basal Stalk Rot, Sclerotinia Mid-stem Rot and Sclerotinia Head Rot
(Sclerotinia sclerotiorum)
Sclerotinia wilt/basal stalk rot, Sclerotinia mid-stem rot and Sclerotinia head rot often are treated as three different diseases, even though they are caused by the same fungus. Sclerotinia head rot is considered the most prevalent and economically important among the sunflower diseases in the northern Great Plains.
Sclerotinia wilt and Sclerotinia mid-stem rot frequently occur and can cause yield loss. In the central high Plains and southern Great Plains, Sclerotinia wilt is the most common and economically important of the three diseases, but mid-stem rot and head rot can occur in prolonged periods of cool and wet weather.

- Figure 106. Sclerotinia wilt. (Sam Markell, NDSU)
.jpg)
The fungus has an extensive host range and is capable of attacking all broadleaf crops and broadleaf weeds. It survives as irregularly shaped, very hard and black structures called sclerotia that commonly resembling rat droppings. Sclerotia are soil-borne and can persist for many years. All sunflower fields in the northern Great Plains are at risk for the disease, even if sunflowers have not been planted previously.
Sclerotinia wilt/basal stalk rot. The disease cycle begins when the soil-borne sclerotia germinate to form mycelium (filamentous fungal strands), which can directly infect growing root tissue of the sunflower. Symptoms manifest weeks later as a cream, tan or light brown lesion at the that soil line girdles the stem (Figures 106-107).
In humid conditions, dense white fungal growth may be apparent on the lesion. Infected plants wilt and/or lodge as they enter reproductive growth stages. Infected plants may occur singly, in a row or in a cluster.

- Figure 107. Sclerotinia wilt basal lesion. (Sam Markell, NDSU)
.jpg)
Sclerotinia mid-stem rot. The disease cycle begins when sclerotia germinate to form a small (1/4 inch in diameter) mushroomlike structure called an apothecium. Sclerotia are environmentally sensitive and may germinate only when they previously have been exposed to cold conditions (winter), are in the top 1 to 2 inches of the soil profile and soil moisture is near saturation.
Ascospores are produced in apothecia, which are liberated and aerially dispersed. Infection begins when ascospores begin colonizing cast flower petals or other decaying plant material on the leaf tissue; ascospores do not infect healthy green tissue directly.
Infection is favored by prolonged periods of free moisture and cool temperatures (75 to 78 F). Once established, the pathogen will spread through the leaf and petiole into the stem, causing a cream to tan lesion around the petiole.
The lesion will enlarge (greater than 6 inches) and will girdle, shred and break the stem, lodging the plant (Figures 108-109). In humid conditions, white fungal growth may be visible on and inside the lesion. Toward the end of the season, black sclerotia will be visible on and/or inside the stem.

- Figure 108. Sclerotinia wilt and Sclerotinia mid-stem rot. (Sam Markell, NDSU)
.jpg)

- Figure 109. Sclerotinia mid stem rot. (Sam Markell, NDSU)
.jpg)
Sclerotinia head rot. The disease cycle is the same as in Sclerotinia mid-stalk rot, but infection begins when ascospores land on flower petals still attached to the sunflower head. Symptoms first observed are a soft, mushy, brown lesion on the back of the head (Figures 110-111).

- Figure 110. Sclerotinia head rot with early symptoms on back of head. (Sam Markell, NDSU)
.jpg)

- Figure 111. Sclerotinia head rot showing progression of symptoms. (Sam Markell, NDSU)
The lesion will enlarge to cover a quadrant or the entire head, and white fungal growth may appear on the face of the head (Figures 112-114).
As the disease progresses, the head will become skeletonized and may be decapitated (Figure 115).
Abundant sclerotia are produced in and on the sunflower head. Unlike sclerotia produced in the stem, they vary heavily in shape and size, and a single sclerotia may be large enough to cover the entire face of the head.

- Figure 112. Severe Sclerotinia head rot infection. (Sam Markell, NDSU)
.jpg)

- Figure 113. Sclerotinia head rot with skeletonization of head. (Sam Markell, NDSU)
.jpg)

- Figure 114. Sclerotinia head rot with white fungal growth and black sclerotia on front of head. (Sam Markell, NDSU)
.jpg)

- Figure 115. Sclerotinia head rot decapitation. (Sam Markell, NDSU)
Management of Sclerotinia wilt, Sclerotinia mid-stem rot and Sclerotinia head rot is challenging. Lengthening crop rotations between sunflower crops is only partially effective because sclerotia survive for many years
in the soil and the pathogen attacks all broadleaf crops.
Management of broadleaf weeds and volunteers increases the effectiveness of crop rotation. Production practices that increase canopy density (excessive nitrogen or seeding rate) should be avoided. Selection of hybrids that are less susceptible is critical in areas prone to Sclerotinia-associated diseases.
Foliar fungicide applications are not recommended. Soil-incorporation of mycoparasites for biological control of S. sclerotiorum may be available, but data on their efficacy are lacking..jpg)
Head Diseases
Bacterial Head Rot
(Pectobacterium carotovorum subsp. carotovorum and P. atrosepticum)
Bacterial head rot occurs only sporadically and is not an economically important disease in the northern Great Plains. The bacteria are ubiquitous and are spread by splashing and wind-driven rain.
The bacterium cells can enter the sunflower head only through a wound; thus, plant damage caused by hail, birds, insects, sand blasting or mechanical injury is a prerequisite to infection. Symptoms begin as a watery soft rot on the back of the head that becomes dark brown as the disease progresses.
Slimy masses of bacterial ooze may fill the head, and a putrid smell resembling rotten potatoes will be apparent. Heads may dry out and turn black after periods of dry weather (Figures 116-117). Notably, the same pathogens that cause bacterial head rot also cause bacterial stalk rot. Active management for bacterial head rot is not needed.
.jpg)

- Figure 117. Bacterial head rot. (Michelle Gilley, NDSU)
.jpg)
Rhizopus Head Rot
(Rhizopus stolonifer, R. arrhizus, R. oryzae and R. microsporus)
Rhizopus head rot is rare and sporadic in the northern Great Plains but more common in the warmer central high Plains and southern Great Plains production regions. Rhizopus head rot can cause significant yield loss when it occurs, and yield losses up to 100% have been recorded in the central high Plains.
The causal fungi are soil-borne and ubiquitous. Favorable conditions for the disease include warm, humid conditions and injury to the developing sunflower head. Hailstorms are most likely to facilitate severe epidemics, but bird, insect and other mechanical damage can lead to Rhizopus head rot.
Once damaged, the fungus enters the wounded area and quickly colonizes the head, producing abundant gray, threadlike fungal growths (mycelium) (Figures 118-120). The mycelium may contain small black fungal fruiting bodies that are visible with a hand lens.
The gray mycelium and black specs distinguish Rhizopus head rot from other head diseases. The back of infected heads becomes watery and soft, rots and eventually dries and becomes dark brown.
Limited management tools for Rhizopus head rot are available. Efforts to limit damage to heads from insects (head moth) and birds after bloom may help prevent conditions leading to Rhizopus head rot. Resistant hybrids and efficacious fungicides are not known at this time.
.jpg)
.jpg)

- Figure 120. Rhizopus head rot with gray fungal growth within head. (Bob Harveson, University of Nebraska)
.jpg)
Sclerotinia Head Rot
(Sclerotinia sclerotiorum)
See above section on Sclerotinia wilt, Sclerotinia mid-stem rot and Sclerotinia head rot (Sclerotinia sclerotiorum).
Other Diseases
Sunflowers are impacted with numerous additional diseases not covered in this production guide. These include albugo (white rust), apical chlorosis, aster yellows, bacteria wilt, Botrytis head rot, crown gall, leaf smut, Louisiana broomrape, several additional leaf spot diseases, rust diseases, root rots and stem rots, as well as diseases caused by nematodes and viruses.
Weeds
Joe Ikley
Weeds compete with sunflowers, causing poor growth and yield losses. Yield loss from weed competition depends on weed species, time of infestation, weed density and climatic conditions.
All weeds are competitors. However, in the northern region of the U.S., wild mustard, wild oats and kochia, which grow rapidly early in the season, appear more competitive than foxtail on a per-plant basis.
A comprehensive weed management program consisting of cultural and/or chemical controls is needed to maximize yields. Sunflower is a good competitor with weeds. However, this competitive advantage occurs only after plants are well-established.
The first four weeks after emergence are most critical in determining weed competition damage, so early weed control is essential. Weeds competing longer than four weeks cause important yield loss even if they are removed.
All chemical recommendations for weed control have a U.S. federal label unless otherwise specified. All recommended herbicides for use in North Dakota have federal registration at the time of printing of this publication, and rates listed are label rates. For up-to-date information, consult the current issue of NDSU Extension publication W253, “North Dakota Weed Control Guide,” which is published annually, or appropriate Extension publications from other states for current labeled products, rates and method of application.
Wild mustard (Sinapis arvensis) is a major weed that infests sunflowers. Wild mustard is not controlled by most of the herbicides commonly used in sunflower. Wild mustard emerges early and appears to be most competitive with sunflowers when the early season is cool. The cool condition favors wild mustard, but not sunflower growth. Late sunflower seeding with seedbed tillage to control early emerged wild mustard can reduce infestations. However, wild mustard may continue to emerge with timely rains and remain a problem even with late seeding. Wild mustard can be controlled easily in Clearfield sunflower with Beyond (imazamox) and in ExpressSun sunflower with Express (tribenuron). Wild mustard is controlled effectively by herbicides used in other crops in the rotation.
Wild oat (Avena fatua) is another cool-season weed that is abundant in North Dakota and causes important yield losses, especially in early seeded sunflower. Wild oat germinates early in the spring, and germination and emergence generally stop when the soil becomes warm. Delayed seeding reduces wild oat infestations. Wild oat can infest late-seeded sunflower when cool and moist conditions occur at or after seeding. Wild oat is controlled to various degrees by several registered herbicides (Table 12).
Table 12. Relative effectiveness of herbicides for various weeds.
|
Herbicide |
Green/yellow foxtail |
Wild oat |
Wild buckwheat |
Common cocklebur |
Kochia |
Common lambsquarters |
Marshelder |
Wild mustard |
Nightshade spp. |
Pigweed/waterhemp |
Russian thistle |
Biennial wormwood |
Canada thistle |
|
Preplant herbicides |
|||||||||||||
|
Glyphosate |
E |
E |
P-G |
E |
F-E1 |
P-E1 |
G-E |
G-E |
P-G |
P-E1 |
G |
F-E |
G-E |
|
Paraquat |
G |
G |
F |
F-G |
G-E |
E |
G |
E |
G-E |
E |
E |
- |
P |
|
Soil-applied herbicides |
|||||||||||||
|
Eptam (EPTC) |
E |
G-E |
F |
P |
P |
F |
P |
P |
F |
F-G |
P |
N |
N |
|
Prowl |
E |
P |
P |
N |
P |
F-G |
N |
N |
N-P |
G-E |
F-G |
N |
N |
|
Sonalan |
E |
P |
P |
N |
P |
F-G |
N |
N |
N-P |
G-E |
F-G |
N |
N |
|
Dual Magnum (s-metolachlor) |
F-E |
P-F |
N-P |
N |
N-P |
P-F |
N |
N |
N |
F-G |
P |
N |
N |
|
Spartan |
P |
N |
P-F |
P |
F-E |
G-E |
P-G |
P |
F-E |
F-E |
G-E |
G |
N |
|
Trifluralin |
E |
P |
P |
N |
F |
G |
N |
N |
N-P |
G-E |
F-G |
N |
N |
|
Zidua (pyroxasulfone) |
G-E |
F-E |
P |
P |
F |
P |
P |
P |
F-G |
G |
F |
F |
N |
|
POST-applied herbicides |
|||||||||||||
|
Assure (quizalofop) |
F-E1 |
G-E1 |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
|
Beyond* (imazamox) |
G-E1 |
E1 |
P |
G-E |
E1 |
F |
G-E |
E |
E |
E1 |
G-E1 |
P |
N-P |
|
Express** (tribenuron) |
N |
N |
P |
N-F |
E1 |
P-F |
E |
E |
P-F |
F-E1 |
E1 |
P-F |
G |
|
Poast (sethoxydim) |
E1 |
G-E1 |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
|
Select (clethodim) |
E1 |
E1 |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
|
* = Clearfield sunflower. |
|||||||||||||
The ratings in the table indicate relative effectiveness, with effectiveness of each herbicide varying with environment and method of herbicide application.
Green foxtail (Setaria viridis) and yellow foxtail (Setaria pumila) are the most abundant grassy weeds in North Dakota. Green and yellow foxtail occur throughout the state. Green foxtail has been more abundant, but yellow foxtail is the dominant species in many areas because herbicides giving less control of yellow foxtail have been used in other crops grown in North Dakota. The two species have similar appearance, but yellow foxtail has a flat stem with long hairs at the base of the leaves, a more brushlike spike and larger seed. Green foxtail has a round stem with no hair on the leaves. Foxtail is a warm-season plant, and germination and emergence do not occur until the soil reaches 60 F. Many sunflower herbicides give excellent control of foxtail species (Table 12).
Kochia (Bassia scoparia) is considered the worst weed problem of sunflower in North Dakota. Kochia is a highly competitive weed that emerges during cool periods early in the spring or later with warm temperatures and adequate moisture. Most kochia has become resistant to ALS (acetolactate synthase) herbicides and no registered herbicides in sunflower give adequate control. Beyond herbicide in Clearfield sunflower is an ALS herbicide and will not control ALS-resistant kochia. Soil-applied Spartan (sulfentrazone) controls ALS-resistant and susceptible kochia when activated by sufficient moisture after herbicide application. Kochia seeds do not have a long residual life in the soil. Good control of kochia in the crop prior to sunflower emergence or control before seeding will reduce the kochia infestation.
Russian thistle (Salsola tragus) is most common in the drier western areas of North Dakota. Russian thistle germinates throughout the season. Germination is rapid, so light rains anytime will promote a new flush of Russian thistle growth. Competition data on losses from Russian thistle in sunflower are not available. The Russian thistle plants are normally small and competition with sunflower usually is not expected. However, Russian thistle is drought tolerant and losses may be severe, even from a small number of plants under conditions of limited moisture.
Other weeds important in sunflower are wild buckwheat (Fallopia convolvulus), redroot pigweed (Amaranthus retroflexus), waterhemp (Amaranthus tuberculatus), common lambsquarters (Chenopodium album), field bindweed (Convolvulus arvensis), Canada thistle (Cirsium arvense), cocklebur (Xanthium strumarium), marshelder (Iva xanthifolia), biennial wormwood (Artemisia biennis), nightshades (Solanum spp.) and wild sunflower. Some of these weeds are controlled partially by soil-applied triflualin or Sonalan (ethalfluralin), but these products cannot be used in no-till sunflower production because of their soil incorporation requirement. Pre-emergence Spartan controls most small-seeded broadleaf weeds and suppresses wild buckwheat, marshelder and foxtail. However, no herbicides are available for selective control of wild buckwheat, Canada thistle, field bindweed, cocklebur, marshelder or wild sunflower. Beyond in Clearfield sunflower controls most annual grass and broadleaf weeds except ALS-resistant weeds, including kochia, but has no activity on perennial broadleaf weeds. Weeds for which no herbicides are available need to be controlled in previous crops in the rotation, or through tillage or the use of herbicides in or between other crops in the rotation.
The sunflower yield loss from individual weeds varies with the weed species, environment and time of weed emergence relative to the crop. Sunflower yield losses from several weeds at various infestations are presented in Figure 121. The values are averages from several years and losses from an individual weed would vary with conditions. A weed that emerges before the sunflowers would be more competitive than one emerging after sunflower establishment, and an environment that favored the growth of the weed would cause a greater loss than if the environment favored the sunflower.
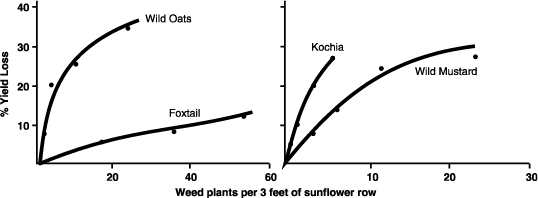
- Figure 121. Percent reduction in sunflower seed yield from several weeds. (J.D. Nalewaja)

Weed Management
Cultural
Cultural weed control requires an integrated system of tillage operations. Weeds must be controlled in other crops in the rotation to reduce the potential infestation level in sunflower.
Preplant, pre-emergence and postemergence tillage practices all must be followed for effective weed control using only tillage. Poor timing or missing any tillage operation can reduce the effectiveness of the cultural weed control program drastically.
Preplant tillage can control one or more weed flushes. Sunflower should be planted immediately after the last tillage operation so the crop can germinate rapidly and compete more favorably. Weeds frequently emerge before sunflower, especially during cool weather. These weeds can be controlled by pre-emergence harrowing.
Postemergence mechanical weed control consists of harrowing and cultivating. Small weeds can be controlled by harrowing after the sunflowers are in the four- to six-leaf stage (V4 to V6) and can resist burial and breaking. Postemergence harrowing should be done across rows and preferably on a warm, clear day to assure sufficient weed kill with the least damage to the sunflower.
Sunflower seedlings, which are strongly rooted, can be harrowed three to five times during the four- to six-leaf stage (V4 to V6). The harrow should be kept free of trash. Spring tooth harrows are recommended; solid spike-tooth harrows should not be used because excessive damage may result.
The direction of travel during harrowing is determined by considering the stand, weed growth and herbicide treatment. Harrowing diagonally to the rows will give better in-the-row weed control than with the row harrowing. However, sunflower damage will occur from the tractor wheels with diagonal harrowing.
Harrowing may be necessary if a soil-applied herbicide was not activated by rainfall, if a field previously treated with a herbicide has weeds resistant to the herbicide or if adverse climatic conditions reduce herbicide effectiveness. If the herbicide is band-applied, harrowing should be parallel to the rows to prevent dilution with untreated soil. A rotary hoe also is effective for postemergence weed control, but weeds must be just emerging for good control.
Setting the harrow or “weighting” the rotary hoe to do the most damage to weeds and the least damage to sunflowers can be accomplished on a “try-and-adjust” system. Postemergence harrowing will kill some sunflowers (5% to 8% loss can be expected), so if this system of weed control is planned, the sunflower should be seeded at higher rates than normal.
After postemergence harrowing, weed control for the remainder of the season depends on the row-crop cultivator. During the first cultivation, producers must take care not to cover the sunflowers. One to three or more cultivations may be necessary, depending on the weed situation in the field.
Lateral sunflower roots are shallow and can be damaged easily by cultivating too deeply and too closely to the plants. Cultivation should be no closer to the row center than the leaf spread of the plants. During later cultivations, soil may be thrown into the row to bury weed seedlings and provide the sunflowers extra support.
Chemical
The most effective weed management is accomplished by an integrated system that uses cultural and chemical control. Preplant cultural practices to reduce weed seed populations, pre-emergence tillage and postemergence cultivation may be needed to supplement the herbicides under adverse climatic conditions and to control late-emerging weeds or weeds that are not controlled by herbicides. Herbicides vary in their effectiveness against various weeds (Table 12).
Refer to the herbicide label or the most current edition of the “North Dakota Weed Control Guide,” NDSU Extension publication W253, for rates, adjuvants and application guidelines.
Preharvest Application
Gramoxone (paraquat) at 0.3 to 0.5 pound (lb) active ingredient per acre (ai/A) can be used as a harvest aid in sunflower. Application should be made when the backside of the sunflower heads is yellow, bracts are turning brown and seed moisture is less than 35%. Apply with a nonionic surfactant at 1 to 2 pints per 100 gallons of water. A seven-day interval must elapse between application and harvest. Paraquat is a restricted-use herbicide.
Sharpen (saflufenacil) at 2 fluid ounces per acre (fl oz/A) can be used as a harvest aid in sunflowers. Application should be made when the backside of the sunflower heads is yellow, bracts are turning brown and seed moisture is less than 35%. Apply with a methylated seed oil (MSO) adjuvant at 1 to 1.5 pints per acre (pt/A) plus ammonium sulfate (AMS) at 8.5 to 17 lbs per 100 gallons of water or urea ammonium nitrate (UAN) at 2.5 gallons per 100 gallons of water. A seven-day interval must elapse between application and harvest.
Valor (flumioxazin) at 1.02 to 1.53 oz ai/A can be used as a harvest aid in sunflower. Application should be made when the backside of the sunflower heads is yellow, bracts are turning brown and seed moisture is less than 35%. Apply with an MSO adjuvant at 2 pt/A plus AMS at 8.5 to 17 lbs per 100 gallons of water or UAN at 2.5 gallons per 100 gallons of water. A five-day interval must elapse between application and harvest.
Glyphosate Preharvest Application in Sunflower
There are some supplemental labels allowing certain applications of glyphosate (Roundup) for control of annual and perennial weeds in sunflower. Apply no more than a total of 0.75 lb of a labeled glyphosate at preharvest. See label for rates suggested.
For preharvest use in sunflower, apply for weed control, not crop desiccation when sunflower plants are physiologically mature. Apply when the backsides of sunflower heads are yellow and bracts are turning brown and seed moisture is less than 35%.
Generally, the dry chaffy material from the disk flowers on the head can be rubbed off easily by hand and expose the seeds at this stage of maturity. Allow a minimum of seven days of preharvest interval (PHI) for sunflower following application.
For postharvest weed control, the products may be applied after the harvest of sunflower. Higher rates may be required for control of large weeds, which were growing in the crops at the time of harvest. Tank mixtures with 2,4-D or dicamba may be used after harvest.
Always follow the pesticide label when applying any product to sunflower.
Herbicide drift is the movement of herbicide from target areas to areas where herbicide application was not intended. Herbicide drift generally is caused by movement of spray droplets or by movement of herbicide vapors. Herbicide granules or dried particles of herbicide may move short distances in high winds but are not considered important sources of herbicide drift.
Sunflower is susceptible to many of the postemergence herbicides commonly used on crops grown in proximity to sunflower For information, see the current NDSU weed control guide, W253.
Birds
Page Klug
Sunflower, due to the easy accessibility and high nutritional value of their seed, is particularly vulnerable to damage by birds (Figure 122). Seeds are exposed and the large head serves as a perch during feeding. Sunflower seed is a preferred bird food because the seed contains many proteins and fats essential to bird growth, molting, fat storage and weight maintenance.
Although many species of birds feed in maturing sunflower, the greatest losses are caused by migrating flocks of red-winged blackbirds, yellow-headed blackbirds and common grackles (Figure 123). Significant losses can occur in fields near cattail marshes.


- Figure 122. Sunflowers may be depredated by birds. Birds perch on sunflower heads and pluck the seeds. (Mallory White, USDA Wildlife Services)
.jpg)
.jpg)


- Figure 123. The red-winged blackbird is the most serious bird pest of sunflower in the northern Great Plains. (USDA Wildlife Services)
.jpg)
Migrating and Feeding Habits of Blackbirds
The adult male blackbird is the first of his species to arrive in the spring. He establishes a territory and awaits the arrival of the females. As females arrive, they disperse to the males’ territories and breeding takes place.
Each female produces a clutch of three or four eggs. Nests are built in dense vegetation, most often in cattails, which offer an abundant food supply.
Following nesting in July, blackbirds begin to form large flocks and can be seen feeding in grain fields. Blackbirds start feeding on sunflower soon after the petals begin to wilt, with damage continuing until harvest in October.
Peak concentrations of blackbirds occur in early October in North Dakota (Figure 124). Most often, birds roost in cattail marshes at night and move to the field for feeding during the day, using smaller marshes and trees as loafing sites.

- Figure 124. Blackbirds cause damage starting in early to mid-September until the end of October or harvest. (Conor Egan, USDA Wildlife Services)
.jpg)
Blackbirds feed on insects and weed seeds in small grain, corn or sunflower fields before these crops are vulnerable to damage. Birds may become used to feeding in a certain location and begin to include sunflower seeds in their diets as the crop matures.
Efforts made by the producer to move birds from a field often are unsuccessful because the birds are in the habit of feeding there. Birds are kept out of sunflower fields most successfully by starting methods to frighten them as soon as the birds are seen in the vicinity, regardless of their diet.
Management
Blackbirds are protected under the Migratory Bird Treaty Act. However, Section 21.43, Title 50 CFR, provides: “A federal permit shall not be required to control yellow-headed, red-winged, tri-colored red-winged, and Brewer’s blackbirds, cowbirds, all grackles, crows and magpies when found committing or about to commit depredations upon ornamental or shade trees, agricultural crops… .” Cultural practices in combination with mechanical and chemical harassment practices should be used to control blackbird damage.
Cultural Practices
A combination of cultural practices may be used to reduce the risk of bird damage to sunflower. If possible, sunflower should not be planted near cattail marshes or woodlots. Unplanted trails allow access to fields for scaring blackbirds from the center of the field. Planting should be done at the same time as neighbors because isolated earlier and later ripening fields suffer more damage.
Weed and insect control should begin early. Insects and weeds in the crop are often an attractive food source for blackbirds before the crop reaches a susceptible stage. Once blackbirds have started feeding in insect-infested or weedy fields, they will include the maturing cultivated crops in their diet when available.
If possible, land preparation after other crops should be delayed until after the sunflower harvest. Crop stubble serves as an alternate feeding area for harassed birds and other wildlife. Sunflower should be harvested as early as possible to avoid prolonged damage periods. Desiccation to the advance harvest also will reduce exposure to birds.
Cattail Management
Dense cattail marshes serving as roosting sites for blackbirds can be managed with a registered aquatic herbicide (for example, glyphosate) to remove cattails used by these birds. Generally, cattails must be treated one year before sunflower is planted in the vicinity of the marsh to allow time for the cattails to decompose. However, herbicide applications made in mid-July might reduce blackbird use of the marsh in the year of application.
The herbicide should be applied from mid-July to late August to at least 70% of the marsh with an agriculture spray plane or helicopter. Use 2 quarts of herbicide per acre. Managing these marshes reduces blackbird use and improves the habitat for other more desirable wildlife, such as waterfowl.
Decoy Crops
Blackbirds can be attracted readily to small plots of oilseed sunflower or other desirable feed crops planted near traditional wetland and tree roost sites. This strategy can be effective for the protection of high-value confectionery and oilseed hybrids.
The plots must produce sufficient seed to feed the expected population of blackbirds. Each bird can eat about 1 pound of sunflower seeds in a season. Thus, if a grower expects 30,000 blackbirds, then a 20-acre plot must produce about 1,500 pounds per acre to feed the birds. These plots also provide essential food and cover for other migrating and game birds.
Automatic Exploders
Automatic exploders or bird-scaring cannons automatically detonate a gas to produce an extremely loud explosion (Figure 125). These devices range from relatively simple mechanisms to deluxe models with photoelectric regulators and programmable firing sequences.
The device should be operated before birds begin to arrive from their roosting area at sunrise and continued as long as birds are in the field. It should be shut off at night. The exploder should be placed on a stand above the crop and it should be adjusted to fire slowly, about every four to five minutes.
The exploder should be moved every two or three days because birds will become accustomed to the noise if operated in the same location day after day. One exploder can protect 10 to 20 acres, especially if used with other mechanical devices and shooting.


- Figure 125. A gas exploder, when properly located and moved within the field every two to three days, can reduce bird damage.(USDA Wildlife Services)

.jpg)
Pyrotechnic Devices
These include cracker-shells, flares, whistlers (fired or pistol launched) and firecrackers. Most of these products are effective in startling birds and are commonly used by many growers. These devices must be used with care, however, because of the potential for mishaps.
Safety glasses and hearing protection are strongly recommended because these devices occasionally detonate prematurely. Pyrotechnics also may be a fire hazard during dry periods.
Shotgun or Rifle
These tools are costly and ineffective as a direct control device. Killing a few birds has little if any direct effect on the rest of the flock. However, shotguns or rifles (only where legal and safe) can be used to reinforce automatic exploders and pyrotechnic devices.
Electronic Frightening Devices
Devices that broadcast distress calls of blackbirds are marginally effective and their application is somewhat limited because of their high cost and limited broadcast range. Furthermore, because they require extensive use of batteries, sophisticated electronic equipment and loud speakers, they are subject to vandalism and theft.
Unmanned Aircraft Systems (Drones)
Harassing feeding blackbirds with drones can be a marginally effective method of chasing flocks from sunflower fields, especially with smaller flocks in smaller fields. The hazing response may be enhanced if combined with other mechanical methods, such as shotguns and pyrotechnic devices. Check with local authorities for permits needed to conduct low-level flying.
Repellents
Environmental Protection Agency-registered repellents for foliar application near harvest are formulated with the active ingredient methyl anthranilate and include various product names (for example, Bird Shield, Avian Control). Research indicates that birds must come into contact with the repellent for it to be effective, making its use limited in sunflower due to application difficulties in getting the product to reach the seeds of the downward-facing sunflowers.
Best results are obtained by using an integrated pest management system that includes habitat management, controlling insects and weeds that might attract blackbirds prior to sunflower ripening, and by using a combination of harassment devices, which must be operated when the birds are in the field.
North Dakota/South Dakota Wildlife Services, telephone 701-250-4405, is a unit within the U.S. Department of Agriculture’s Animal and Plant Health Inspection Service (USDA-APHIS). It operates a cost-share blackbird management program in North Dakota and South Dakota.
The North Dakota Field Station of the USDA-APHIS Wildlife Service National Wildlife Research Center, telephone 701-231-5190, conducts research to test and develop methods and tools to manage bird damage to crops.
Other Pests and Damage
Hans Kandel
Rabbits
Rabbits will start foraging soon after seedling emergence, especially near the edges of fields. They will tend to concentrate on one row and apparently eat their fill, then leave until the next feeding period. Continued feeding by rabbits has been observed until the plants are 8 to 10 inches tall. Rabbit feeding on such large plants may be confused with deer. However, deer can be detected by their tracks.
Deer
Deer begin foraging on sunflower plants when the plants reach 8 to 10 inches and continue through harvest. They feed in areas near cover, such as wooded areas. All leaves of young plants will be consumed below the growing point. Heads will be foraged until near maturity and seeds until harvest. Often deer will knock down the stalk to facilitate foraging.
Gophers and Mice
Gopher and mouse damage usually is seen just after planting. It generally occurs next to overgrazed pastures, grassland recently converted to cropland and fields next to abandoned areas. The seed will be dug up, split open with the kernel consumed and the hull left on the soil. Several seeds in a row will be eaten. Seedlings are eaten occasionally when they are 2 to 3 inches tall. If the growing point is consumed, the seedling gradually dies.
Lightning
Lightning damage sometimes is mistaken for a disease. It is distinguished from disease damage by the sudden death of the plants in the affected area and the fact that sunflowers and weeds (not grass, however) are killed. Near the edge of the area, plants are wilted but not dead, and the stalks may have a brown to blackened pith. The area may be as large as 50 to 100 feet in diameter. The affected area usually is circular and does not increase in size after the first two weeks.
Flooding
Soils should have good drainage for sunflower production, but the crop doesn’t differ greatly from most other crops. In flooded sunflower, research found that ethylene increased in the stems and roots below the water. Later, chlorophyll breakdown and leaf epinasty resulted.
Sunflower plants flooded longer than three days may not recover. Cool, cloudy days during the flooding period reduce the damage, whereas hot and sunny days may hasten the death of plants.
Heat Canker
Warm temperatures and sunny days can result in heat canker injury to young sunflower seedlings growing in black or dark, moist soils. Hot temperatures at the soil line cause cell death in the young stem and the plants will show bands of yellowing and constricting. In severe cases, the constricted area completely girdles the stem at the soil line and the plant topples over. The sunflower seedling will not recover because the growing point is above this site.
Frost Damage
Sunflower seedlings in the cotyledonary stage (VE) can withstand temperatures down to 26 F when just emerging from the soil. Sunflowers in the V1, V2 and V3 stages become less tolerant to frost as they grow and develop. The terminal bud can be frost damaged in seedlings with two, four and six true leaves. This early frost damage and killing of the terminal bud can result in excessive branching as the sunflowers grow and develop.
Sunflowers are most susceptible at the bud (R4) and pollination stages (R5.0 to R5.9) of development. Temperatures of 30 F or less can cause damage to the anthers and stigmas of the pollinating disk flowers. (See Figure 126 for frost-damaged sunflower head).
.png)
Sunflower has a composite type flower. Several rows of showy yellow ray flowers encircle the head and commonly are called the “petals,” although each is an individual flower. The center portion of the head, and by far the greater part, is composed of inconspicuous individual flowers, one for each seed that may develop.
These disk flowers mature in circles from the outside of the flower head to the center, so that at various stages, the disk flowers ready for pollination appear as a yellow circular band in the brownish or dark center of the head. These disk flowers are sensitive to frost.
The result of the frost damage in the flowering period is circular bands of undeveloped seed that would vary with individual flower heads from a band around the outside edge to an area in the center. Unopened buds are less susceptible to frost than the opened flower heads. Growers can determine the extent of injury by cutting the surface of the flower head.
Once pollination is completed and 10 to 14 days after petal drying occurs, the sunflower plants can withstand frost temperatures as low as 25 F and have only minor damage. If hard frosts do occur, many times only the seed in the center of the head (the last to pollinate) will be affected.
When sunflower heads start to turn yellow on the backside and the bracts are drying and turning brown, most risk of frost damage is very minimal.
In nonoilseed sunflower, frost damage can cause quality problems by causing a dark brown to blackened nutmeat to result during the roasting process. For the birdseed market, light-weight sunflower seed and brown seeds are the result of frost damage and will be discounted. For oilseed sunflower, reduced test weight per bushel and lower oil percent may result from a frosted immature sunflower crop.
Hail Injury
Hans Kandel
Hail storms can and will cause different types of sunflower plant injury. Plant death, damage to the terminal bud, physical injury to the stalk and head, and defoliation are all types of injury that can influence yield.
Variables such as hailstone size and degree of hardness, speed and density, storm duration and plant environmental status, such as whether the leaves are flaccid or turgid, influence the type and degree of crop injury. The stage of plant development is also an important factor (Figure 127).

- Figure 127. Sunflowers defoliated by hail. (Albert Schneiter, NDSU)
.jpg)
One of the major factors causing differential growth and yield response is the stage at which the injury occurred. Data were obtained from a sunflower date-of-planting study at Carrington, N.D.
Five sunflower hybrids sown at six planting dates between May 1 and June 20 were damaged by a hail storm on Aug. 6. Stages of plant development at the time of the storm were from R1 to R7. Data were taken approximately one week after the storm.
The average percent of defoliation from all planting dates was similar at about 26%. An average of 4.7 stalk and head stone bruises occurred per nondestroyed plant. The percent of plants destroyed and the percent of the remaining plants with heads broken off or bent over but attached decreased with plant maturity and is provided in Table 13.
Table 13. Effect of hail injury on sunflower at several stages of plant development.
|
Date |
Approx. |
|
Injury on nondestroyed plants |
||
|
% Plants |
% Heads |
% Heads |
% |
||
|
May 1 |
R7 |
19 |
8 |
14 |
78 |
|
May 9 |
R6 |
24 |
6 |
17 |
77 |
|
May 21 |
R5 |
24 |
17 |
18 |
65 |
|
May 30 |
R3 |
30 |
16 |
17 |
68 |
|
June 10 |
R2 |
56 |
36 |
24 |
40 |
|
June 20 |
R1 |
40 |
23 |
38 |
61 |
Defoliation: Reduced yield as a result of defoliation depends on the amount of leaf loss and the stage at which it occurs. Stages R1 through R6 appear to be the most sensitive to defoliation because much of the photosynthate produced at this time is directed to head development. At early and late stages of plant development, high levels of defoliation may not have a major impact on seed yield. Approximate yield reductions due to varying degrees of random defoliation at several stages of growth are presented in Table 14.
Stand Reduction: Plant death as a result of hail injury is a common occurrence, especially at early stages of development when plants are small. At early stages of plant development, before plants begin competing with each other, yield losses due to stand reduction caused by hail are not different than those that would occur due to reduced seeding rates.
If the amount of stand reduction is significant and/or occurs when the plant has begun to develop and compete with neighboring plants, the remaining uninjured plants cannot compensate enough and yields will be reduced. Losses due to stand reduction increase as the plant matures because it decreases the time for remaining plants to compensate.
Table 14. Approximate percent yield reduction from the indicated percent total leaf area destroyed at several stages of sunflower plant development.
|
Stage1 |
Percent Leaf Area Destroyed |
|||||||||||||||||||
|
5 |
10 |
15 |
20 |
25 |
30 |
35 |
40 |
45 |
50 |
55 |
60 |
65 |
70 |
75 |
80 |
85 |
90 |
95 |
100 |
|
|
- - - - - - - - - - - - - - - - - - - - Approximate percent yield loss - - - - - - - - - - - - - - - - - - - - |
||||||||||||||||||||
|
VE to V3 |
0 |
0 |
0 |
1 |
1 |
1 |
2 |
2 |
2 |
3 |
3 |
3 |
4 |
4 |
5 |
7 |
8 |
10 |
12 |
15 |
|
V4 to V5 |
0 |
0 |
0 |
1 |
2 |
2 |
2 |
2 |
3 |
4 |
4 |
4 |
5 |
5 |
7 |
9 |
12 |
14 |
17 |
21 |
|
V6 to V8 |
0 |
0 |
0 |
1 |
2 |
2 |
2 |
2 |
3 |
4 |
4 |
5 |
6 |
6 |
8 |
10 |
14 |
16 |
19 |
22 |
|
V9 to V11 |
0 |
0 |
1 |
2 |
3 |
3 |
4 |
4 |
4 |
5 |
5 |
5 |
5 |
7 |
9 |
11 |
14 |
17 |
21 |
24 |
|
V12 to V(N) |
0 |
1 |
2 |
3 |
4 |
4 |
5 |
5 |
5 |
6 |
7 |
7 |
9 |
12 |
15 |
18 |
22 |
26 |
31 |
35 |
|
R1 |
0 |
2 |
3 |
4 |
5 |
6 |
6 |
6 |
7 |
7 |
8 |
9 |
13 |
16 |
20 |
24 |
29 |
34 |
40 |
47 |
|
R2 |
0 |
2 |
3 |
4 |
6 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
16 |
18 |
23 |
30 |
37 |
45 |
55 |
65 |
|
R3 |
0 |
2 |
5 |
8 |
10 |
15 |
17 |
19 |
21 |
24 |
28 |
32 |
38 |
44 |
51 |
59 |
68 |
78 |
88 |
99 |
|
R4 |
0 |
2 |
4 |
5 |
7 |
10 |
12 |
12 |
15 |
18 |
22 |
27 |
34 |
39 |
45 |
53 |
61 |
72 |
85 |
99 |
|
R5 |
0 |
1 |
2 |
3 |
5 |
7 |
8 |
10 |
13 |
16 |
20 |
25 |
32 |
37 |
43 |
49 |
55 |
67 |
78 |
90 |
|
R6 |
0 |
0 |
1 |
1 |
3 |
3 |
4 |
8 |
11 |
15 |
19 |
24 |
29 |
35 |
41 |
46 |
53 |
63 |
72 |
80 |
|
R7 |
0 |
0 |
1 |
1 |
1 |
3 |
5 |
7 |
8 |
10 |
11 |
13 |
14 |
16 |
17 |
18 |
19 |
20 |
21 |
22 |
|
R8 |
0 |
0 |
1 |
1 |
1 |
2 |
2 |
3 |
4 |
5 |
6 |
7 |
7 |
8 |
9 |
9 |
10 |
10 |
10 |
11 |
|
R9 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
1Number of days after planting for development to a specific stage will vary significantly depending on environmental conditions and the hybrid. Interpolating percent of loss between stages may be necessary. |
||||||||||||||||||||
Approximate yield reductions from variable levels of random stand reduction at several stages of plant development are presented in Table 15. These values represent direct stand reduction where the plants have been destroyed and no longer are competing with uninjured plants for light, water or nutrients.
Injured Plants: In addition to stand reduction and defoliation, injuries such as terminal bud removal or injury and stem breakage or bruising may occur as a result of hail. Plants that are injured but living sometimes may reduce total crop yield more than if they had been completely destroyed because they continue to compete with uninjured plants for space, light and nutrients but do not produce an equal yield.
The response of plants to a hail injury, such as terminal bud removal, varies depending on the stage at which the injury occurs. When plants are injured in this manner at vegetative (V) stages, they usually develop branches that produce small seed-bearing heads. When injury to the terminal bud occurs during the early reproductive (R) stages, a greater percentage of the plants may die.
When injury occurs near or after flowering, the plants usually remain green and continue to live but do not produce seed. A similar type of response can be evident when plants have been injured by the head-clipping weevil; however, the injury from the head-clipping weevil is a straight cut across the stalk.
The effect of bruising by hailstones is difficult to determine. If the amount of stalk bruising is such that the plant does not weaken or break during the remainder of its development prior to harvest, the effects on yield may be minimal.
Physical injury by hailstones on the back of a sunflower head at or near anthesis can result in Rhizopus head rot, especially if wet or humid conditions are present. Physical injury can occur as a result of bird, insect or hailstone damage. Increased dead plant tissue resulting from a hail storm, especially on the back of a head, may increase the chance of white mold infection.
Table 15. Approximate percent yield reduction from the Indicated percent stand reduction at several stages of sunflower plant development.
|
Stage1 |
Percent Stand Reduction |
|||||||||||||||||||
|
5 |
10 |
15 |
20 |
25 |
30 |
35 |
40 |
45 |
50 |
55 |
60 |
65 |
70 |
75 |
80 |
85 |
90 |
95 |
100 |
|
|
- - - - - - - - - - - - - - - - - - - - Approximate percent yield loss - - - - - - - - - - - - - - - - - - - - |
||||||||||||||||||||
|
VE to V3 |
0 |
1 |
2 |
3 |
4 |
8 |
10 |
11 |
12 |
12 |
13 |
14 |
16 |
18 |
24 |
32 |
43 |
58 |
77 |
100 |
|
V4 to V5 |
0 |
1 |
2 |
3 |
4 |
8 |
10 |
11 |
12 |
12 |
13 |
14 |
16 |
18 |
24 |
32 |
43 |
58 |
77 |
100 |
|
V6 to V8 |
0 |
1 |
2 |
3 |
4 |
8 |
10 |
11 |
12 |
12 |
13 |
14 |
16 |
18 |
24 |
33 |
43 |
58 |
77 |
100 |
|
V9 to V11 |
0 |
1 |
2 |
3 |
4 |
8 |
10 |
11 |
12 |
12 |
13 |
14 |
16 |
19 |
25 |
33 |
44 |
59 |
77 |
100 |
|
V12 to V(N) |
0 |
1 |
2 |
3 |
4 |
8 |
10 |
12 |
12 |
13 |
14 |
15 |
17 |
21 |
27 |
35 |
46 |
60 |
78 |
100 |
|
R1 |
1 |
2 |
5 |
9 |
12 |
14 |
15 |
16 |
17 |
18 |
19 |
21 |
25 |
29 |
35 |
43 |
53 |
66 |
81 |
100 |
|
R2 |
2 |
3 |
7 |
9 |
13 |
17 |
19 |
21 |
23 |
24 |
26 |
28 |
31 |
35 |
40 |
47 |
57 |
68 |
83 |
100 |
|
R3 |
4 |
7 |
11 |
13 |
15 |
17 |
21 |
24 |
27 |
29 |
31 |
34 |
37 |
41 |
46 |
53 |
61 |
72 |
84 |
100 |
|
R4 |
5 |
10 |
14 |
18 |
20 |
22 |
25 |
27 |
29 |
32 |
35 |
38 |
42 |
47 |
53 |
60 |
68 |
77 |
88 |
100 |
|
R5 |
5 |
10 |
14 |
19 |
20 |
24 |
28 |
31 |
35 |
39 |
42 |
45 |
49 |
54 |
60 |
66 |
73 |
81 |
90 |
100 |
|
R6 |
5 |
10 |
15 |
19 |
22 |
26 |
31 |
35 |
39 |
44 |
48 |
52 |
56 |
62 |
68 |
73 |
79 |
85 |
93 |
100 |
|
1Number of days after planting for development to a specific stage will vary significantly depending on environmental conditions and hybrid. It may be necessary to interpolate percent of loss between stages. |
||||||||||||||||||||
Harvesting
John Nowatzki
Maturity
The sunflower plant is physiologically mature when the back of the head has turned from green to yellow and the bracts are turning brown (Stage R9), about 30 to 45 days after bloom, and seed moisture is about 35%. Desiccants can be applied to the crop after physiological maturity to speed the dry-down process. The chemical compounds act much like a frost to kill the green tissue on the plant and accelerate its drying.
After applications of a desiccant, dry down of the seed is not as rapid as the dry down of the plant. Application of a desiccant before the plant reaches physiological maturity will reduce yield and lower oil percentage. Seed shattering loss during harvest and loss from birds may be reduced by harvesting sooner when the sunflower seed moisture content is as high as 25%.
Harvesting Attachments
Combines suitable for threshing small grains can be adapted to harvest sunflower. A variety of header attachments are available, with many operating on a head stripper principle.
The attachments are designed to gather only the sunflower heads and eliminate as much stalk as possible. Major components of this attachment are catch pans, a deflector and a small reel. Long catch pans extend ahead of the cutter bar to catch the seed as it shatters.
The deflector mounted above the catch pans pushes the stalk forward until only the heads remain above the cutter bar. As the heads move below the deflector, the stems contact the cutter bar and are cut just below the head. A small reel, mounted directly behind the deflector, pushes the heads into the combine feeder.
Catch pans are available in various widths. These range from narrow 9-inch pans spaced on 12-inch centers to 37-inch pans spaced on 40-inch centers. The narrow 9-inch pans can operate on any row spacing, while the wider and more efficient 30- to 40-inch spaced pans are limited to a fixed-row spacing.
Lift rods are available to bolt to most headers with catch pans. The lift rods are effective when harvesting lodged sunflowers.
The deflector consists of a curved piece of sheet metal the full width of the combine head. It is attached to the reel support arms above the catch pans. The reel for the unit is mounted directly behind the deflector and usually consists of three or four arms.
The reel is usually 16 to 20 inches in diameter and mounted 4 to 5 inches above the catch pans so when the heads come in contact with the reel, they are pushed back into the feeder. The shield and reel can handle tall plants while taking only a minimum length of stalk with the head, allowing harvest when the seed is dry but stalk moisture may remain above 50%.
Optional forward rotating stalk-walker shafts can be mounted under the cutter bar to reduce plugging of stalk slots between pans. The stalk-walker pulls sunflower stalks and weeds down so that only the sunflower head is fed into the combine.
A rotating drum with metal projections that replaces the deflector bar and reel often is used. The projections are triangular-shaped pieces of strap iron welded to its surface. As the drum rotates, the projections pass through the slots between the catch pans to remove any stalks that may cause clogging. The smooth drum acts as a deflector bar to strip stalks until one of the projections catches a head and pushes it into the cutter bar and into the combine header.
Row-crop units mounted on combine headers have been used successfully to harvest sunflower seed. One unit uses gathering belts, one on each side of the row, to draw the stalk into the cutting unit and the header. A large quantity of stalk passes through the machine with this unit and may increase the foreign matter in the seed, but this unit works well picking up lodged sunflowers and getting the heads into the machine.
Another type of header uses a short section of screw conveyor to pull the stalks into the cutter bar and the combine header. This unit also works well for picking up lodged sunflower.
Combine Adjustments
Cylinder speed: After the sunflower heads are separated from the plant, they should be threshed at a cylinder speed operating as slowly as possible. The normal cylinder speed should be about 300 revolutions per minute (rpm), depending upon the condition of the crop and the combine being used.
This cylinder speed is for a combine with a 22-inch-diameter cylinder to give a cylinder bar travel speed of 1,725 feet per minute. Combines with smaller cylinders will require a faster speed and combines with a larger cylinder diameter will require a slower speed.
Rotary combines, as well as conventional machines, should have similar cylinder travel speeds. A rotary combine with a 30-inch cylinder will need to be operated at 220 rpm to have a cylinder bar speed of 1,725 feet per minute. A combine with a 17-inch cylinder will need to operate at 390 rpm to have a cylinder bar speed of 1,725 feet per minute.
If a combine cylinder operates at speeds of 400 to 500 rpm, giving a cylinder bar speed of more than 2,500 feet per minute, very little seed should be cracked or broken if the moisture content of the seed is above 11%. Cylinder bar speeds of more than 3,000 feet per minute should not be used because they will cause excessive broken seed and increased dockage. Excess dockage and broken seed may overload the sieves and the return elevator.
Concave adjustment: Sunflower threshes relatively easily. When crop moisture is at 10% or less, conventional machines should be set wide open to give a cylinder-to-concave spacing of about 1 inch at the front of the cylinder and about 0.75 inch at the rear. A smaller concave clearance should be used only if some seed is left in the heads. If the moisture percentage of the crop is between 10% and 12%, rather than increase the cylinder speed, the cylinder-to-concave clearance should be decreased to improve threshing.
If seed moisture exceeds 15% to 20%, a higher cylinder speed and a closer concave setting may be necessary, even though foreign material in the seed increases. Seed breakage and dehulling may be a problem with close concave settings. Make initial adjustments as recommended in the operator’s manual. Final adjustments should be made based on crop conditions.
Rotary combines should be set to have a rotor-to-concave spacing of about 0.75 to 1 inch. Making initial settings as recommended in the operator’s manual usually is best. Final adjustments should be made based on crop conditions.
Fan adjustment: Oilseed and nonoilseed sunflower weigh about 28 to 32 pounds per bushel and 22 to 26 pounds per bushel, respectively. The seed is relatively light compared with other crops, so excessive wind may blow seed over the chaffer and sieve.
Seed forced over the sieve and into the tailings auger will be returned to the cylinder and may be dehulled. Only enough wind to keep the trash floating across the sieve should be used. The chaffer and sieve should be adjusted to minimize the amount of material that passes through the tailings elevator.
When the combine is adjusted correctly to thresh sunflower seed, the threshed heads will come through only slightly broken and with only unfilled seed remaining in the head. Cylinder concaves and cleaning sieves usually can be set to obtain less than 5% dockage. Improper settings will crush the seed but leave the hull intact.
Proper setting is critical, especially for nonoilseed sunflower that is used for the human food market. The upper sieve should be open enough to allow an average seed to pass through on end, or be set at a ½- to e-inch opening. The lower sieve should be adjusted to provide a slightly smaller opening, or about d inch wide.
The final adjustments will depend on the amount of material returning through the tailings elevator and an estimation of the amount of dockage in the grain tank. Some operators are able to adjust and operate their machine to allow only 2% to 3% dockage in the seed.
Field Loss
The harvested yield of sunflower can be increased by making necessary adjustments following a determination of field loss. Three main sources of loss are: (a) loss in the standing crop ahead of the combine, (b) header loss as the crop enters the machine and (c) threshing and separating loss. The loss found in any of these three areas will give the combine operator a good estimate of sources of seed loss and the adjustments necessary to minimize seed loss.
Loss occurring in any of these areas may be estimated by counting the seed on the soil surface in a square-foot area. Ten seeds per square foot equal approximately 1 hundredweight (cwt) per acre loss if seed loss is uniform throughout the entire field.
The loss in the standing crop is estimated by counting the seed in a 1-square-foot area ahead of the machine at several different places in the field. Header loss can be calculated by counting seed in a 1-square-foot area behind the head under the combine and subtracting the standing crop loss.
The loss in combine separation can be found by counting the seed in a 1-square-foot area directly behind the rear of the combine and subtracting the shatter loss and the header loss found under the machine. The count made directly behind the combine will be concentrated, so an adjustment must be made to equalize the loss over the entire width of cut. The result should be divided by the ratio:
Width of Header Cut (feet) / Width of Rear of Combine (feet)
The answer is the adjusted separator loss for the width of cut. This result must be divided by 10 to obtain the combine separator loss in cwt per acre. The total loss in cwt per acre is determined by adding the seed loss in the standing crop, header loss and separator loss and dividing this answer by 10. The percentage loss can be found by dividing the total cwt per acre by the yield in cwt per acre.
A harvest without some seed loss is almost impossible. Usually a permissible loss is about 3%. Loss as high as 15% to 20% has occurred with a well-adjusted combine if the ground speed is too fast, resulting in machine overload.
Drying and Storage
Kenneth Hellevang
Harvesting sunflower at higher moisture contents normally results in higher yields due to less field loss. Early harvest also reduces exposure to late-season wet and cold weather. Frequently, mechanical drying is required so harvesting can be completed.
Natural-air, low-temperature and high-temperature bin, batch (Figure 128) and continuous-flow dryers can be used to dry sunflower.

- Figure 128. A high-temperature column dryer used for drying sunflower. (Kenneth Hellevang, NDSU)
.jpg)
Natural-air and Low-temperature Bin Drying
Natural-air and low-temperature bin drying is energy efficient if designed properly and permits rapid harvest because bins can be filled at the harvest rate. Drying will take three to six weeks, depending on the initial moisture content, airflow rate and outdoor temperature.
Required airflow rates and drying time for drying oil sunflower at various moisture contents using air at 47 F and 65% relative humidity (average North Dakota conditions for October) are shown in Table 16. Drying times will be twice as long at 27 F (average November temperatures) due to the reduced moisture-holding capacity at colder temperatures. Heating the air more than about 5 degrees normally causes overdrying.
Table 16. Recommended airflow rates and drying times for natural-air drying oilseed sunflower in October (47 F and 65% relative humidity).
|
Moisture Content |
Airflow |
Fan Time |
|
|
Hours |
Days |
||
|
17% |
1.00 |
648 |
27 |
|
15% |
1.00 |
480 |
20 |
|
0.75 |
720 |
30 |
|
|
0.50 |
960 |
40 |
|
|
13% |
1.00 |
336 |
14 |
|
0.75 |
504 |
21 |
|
|
0.50 |
672 |
28 |
|
Add enough heat when needed to dry the sunflower to the safe storage moisture content. Generally, enough heat to warm the air about 5 degrees is the maximum amount required.
As a rule of thumb, about 2 kilowatts (kW) of heat will be required per fan motor horsepower. The equation for calculating the heat requirement in Btu is: Btu/hr = cfm x 1.1 x temperature increase. Convert Btu to kW by dividing by 3,413 Btu/kW.
A perforated floor is recommended. Because air does the drying, making sure air reaches all the sunflower seeds is imperative. The uniform airflow distribution required for drying is more difficult to achieve with ducts than with perforated floors. However, drying can be done successfully if ducts are spaced no more than one-half the grain depth apart and the distance from the duct to bin wall does not exceed one-fourth the grain depth.
Provide 1 square foot of duct or floor perforated surface area for each 50 cubic feet per minute (cfm) of airflow. One square foot of bin exhaust opening should be provided for each 1,000 to 1,500 cfm of airflow.
High-temperature Dryers
Drying temperatures up to 220 F do not appear to have an adverse effect on oil percentage or fatty acid composition. High drying temperatures for the nonoil varieties may cause the kernels to be steamed, wrinkled or even scorched.
Column batch and bin batch dryers should be operated at 180 and 120 F, respectively. Continuous-flow and recirculating batch dryers may be operated at temperatures up to about 200 F. Temperatures more than 110 F should not be used to dry sunflower seed for seeding purposes.
Fire Hazard
Fire hazards exist in dryers used for sunflower. A major concern is that some sunflower seeds or foreign material will accumulate in the dryer and become overdried.
Make sure the dryer is completely cleaned out after each batch, and check a continuous-flow dryer regularly (at least hourly) to see that the sunflower seed is moving. The potential for fires is not related to drying temperature but rather to housekeeping.
Also, fine hairs or fibers from the seed are rubbed loose during handling and may be floating in the air around the dryer. These hairs or fibers or other plant materials may be ignited when drawn through the drying fan and open burner. A fire hazard is present unless these ignited particles burn themselves out before contacting the sunflower seed.
The fire hazard due to the fibers is decreased if the fans of a portable dryer are turned into the wind to draw clean air and by pointing stationary dryers into the prevailing wind.
High-speed dryers are like a forge when a fire gets going. However, fires can be controlled if they are noticed immediately, which makes constant monitoring necessary.
Many fires can be extinguished by just shutting off the fan to limit the oxygen. A little water applied directly to the fire at the early stages may extinguish it. A fire extinguisher for oil-type fires should be used for oil sunflower fires.
Some dryers are designed so that sunflower seeds can be unloaded rapidly in case of a fire, before the dryer is damaged. In some dryers, just the part of the dryer affected by the fire needs to be unloaded.
Measuring Moisture Content
Measuring the moisture content of sunflower seeds immediately after removal from the dryer only provides an estimation. As moisture is removed from the sunflower seed, the hull dries first and the kernels dry last.
Moisture testers used by local grain elevators and farm operators generally result in a reading that is lower than the actual moisture percentage when moisture is measured while the moisture variation exists. The initial moisture content of the sunflower seeds and the temperature of the drying air influence the amount of error.
A number of operators have reported that sunflower seeds removed from the dryer at 9% to 10% moisture (according to the moisture tester) would be up to 12% moisture later. The moisture rebound can be estimated by placing a sample from the dryer in a sealed container and then rechecking the moisture after 12 hours.
Guidelines for drying sunflower are:
- The area around the dryer and the plenum chamber should be cleaned thoroughly.
- A continuous flow of sunflower seeds in all sections of recirculating batch and continuous-flow dryers should be maintained. Uneven flow will cause overdried spots and increase the fire hazard.
- Drying equipment must not be left unattended.
- The dried sunflower seed should be cooled to near outside air temperature before storing.
Storage
Farm structures that are structurally adequate to store other grains are adequate for storing sunflower due to sunflowers’ light test weight. See Figure 129.

- Figure 129. Structures adequate to store other grains are adequate for sunflower. (Kenneth Hellevang, NDSU)
.jpg)
Seed should be cleaned for storage. Fines tend to concentrate in the center of the bin if a distributor is not used. Because this material tends to be wetter, this area is more prone to storage problems.
Also, airflow will be restricted by the fines, limiting cooling by aeration in the center of the bin. Large pieces of head, stalk and corolla tubes, which frequently adhere to the seed, should be removed because they are higher in moisture than the seed.
Oil sunflower should not be stored above 10% moisture during the winter and 8% during the summer. Nonoil sunflower should not be stored above 11% moisture during the winter and 10% during the summer.
Sunflower can be stored for short periods in the fall at 12% with adequate airflow to keep the seeds cool. Resistance of oilseed sunflower to fungal infestation during storage at 10% moisture is equal to wheat resistance at about 15% stored moisture.
Aeration to control seed temperature is essential. Aeration fans normally are sized to provide about 0.2 cfm/bu. (0.6 cfm per cwt) of sunflower (Figure 130). Sunflower seeds should be rotated between bins during the storage period when aeration is not available.

- Figure 130. Aeration is critical for proper storage. (Kenneth Hellevang, NDSU)
.jpg)
.jpg)
Cooling sunflower reduces the potential for sunflower deterioration from insects and mold. Sunflower seeds should be cooled to 40 F or below before or soon after they are put in the bin and to about 25 F for winter storage. Insects become dormant and will not cause damage or multiply if the seed temperature is below about 50 F.
Moisture and heat accumulate in the peak due to moisture migration, which results in crusting, spoilage and increased possibility of insect infestations (Figure 131). This can be prevented by cooling the sunflower seeds using aeration.
- Figure 131. Moisture migration leads to increased moisture in top center of stored sunflower. (Kenneth Hellevang, NDSU)

Bins should be checked initially every two weeks for moisture condensation on the roof, crusting and changes in temperatures within the pile. Any of these conditions could indicate the presence of mold or insects.
If the sunflower seeds have started to heat, they should be cooled immediately. The sunflowers should be checked at least monthly after the seeds have been cooled to about 25 F for winter storage and a history of temperature and moisture content has been developed. As outside temperatures increase, stored sunflowers should be checked at least every two weeks.
Seed lots containing a high percentage of hulled seed or immature seed, such as seed resulting from an early frost, tend to deteriorate in storage, affecting oil quality.
Feeding Value of Sunflower Products in Beef Cattle Diets
Karl Hoppe
Sunflower Meal
Nutrients in sunflower meal can vary depending on several factors. The amount and composition of meal is affected by oil content of the seed, extent of hull removal and efficiency of oil extraction.
The proportion of the hull removed before processing differs among crushing plants. In some cases, a portion of the hulls may be added back to the meal after crushing. The amount of hull or fiber in the meal is the major source of variation in nutrients (Table 17).
Table 17. Nutrient content of solvent-extracted sunflower meal based on amount of hulls retained.
|
Dry Matter, % |
No Hulls |
Partially |
Dehulled |
|
90.0 |
90.0 |
90.0 |
|
|
Percent, Dry Matter Basis |
|||
|
Crude protein |
28.0 |
34.0 |
41.0 |
|
Fat |
1.5 |
0.8 |
0.5 |
|
Crude fiber |
24.0 |
21.0 |
14.0 |
|
Ash |
6.2 |
5.9 |
5.9 |
|
Calcium |
0.36 |
0.35 |
0.34 |
|
Phosphorus |
0.97 |
0.95 |
1.30 |
|
Potassium |
1.07 |
1.07 |
1.07 |
|
Magnesium |
0.80 |
0.79 |
0.79 |
|
Hesley (ed), National Sunflower Association, 1994. |
|||
Sunflower meal is marketed and shipped as meal or pellets. Protein required by rumen microbes can be provided in the form of rumen-degradable protein from sunflower meal.
Heat treatment or toasting of meal from the solvent extraction process may increase the proportion of undegradable protein. Sunflower meal is more ruminally degradable (74% of crude protein) than soybean meal (66%) or canola meal (68%).
Sunflower Meal in Beef Cattle Diets
Sunflower meal can be used as the sole source of supplemental protein in beef rations. In trials comparing sunflower meal with other protein sources, equal animal performance commonly is observed based on isonitrogenous diets from different sources.
Cows consuming low-quality forages, such as winter range, crop aftermath or other low-quality forages, can utilize supplemental degradable protein to increase total intake, forage digestibility and performance. Protein can be supplemented with a number of feeds, coproducts or oilseed meals. Least costly sources are critical to profitability, and sunflower meal often is very competitively priced per unit protein.
Sunflower Silage
Sunflower silage can make a suitable feed for beef cows; however, high moisture levels can be a challenge since sunflowers typically dry down slowly. Consequently, dry feed must be added to the silage pile to reduce the moisture level to a point where seepage is not a major problem.
Table 18 gives the estimated nutrient content of sunflower silage produced from either low-oil or high-oil varieties of sunflower. Depending on what other feeds are mixed in the silage pile, nutrient contents may change.
Blending corn and sunflower silages together can help alleviate the moisture problem. Producers also may consider waiting seven to 10 days following a killing frost to facilitate dry down. Blending dry forage into the silage pile also can reduce moisture content. To minimize seepage problems, the moisture level should be 65% or less.
Whole Sunflower Seeds
When economical, whole sunflower seeds can be used as a source of energy and protein in beef cattle diets (Table 18). Fat levels can be quite high in whole seeds; consequently, amounts fed should be restricted based on fat content of the seed. Typically, no more than 4% supplemental fat should be added to cow diets to reduce the potential for any detrimental effects on fiber digestion. This will result in inclusion levels of approximately 10% of the diet.
Table 18. Nutrient content of sunflower products.
|
DM, % |
TDN, % |
NEm, Mcal/lb |
NEg, Mcal/lb |
CP, % |
ADF, % |
Ca, % |
P, % |
|
|
Sunflower Hulls* |
90.0 |
40.0 |
0.41 |
0.00 |
5.0 |
63.0 |
0.00 |
0.114 |
|
Sunflower Screenings* |
87.0 |
64.0 |
0.66 |
0.39 |
11.1 |
29.0 |
0.72 |
0.42 |
|
Sunflower Seed, Confectionary* |
94.9 |
83.0 |
0.93 |
0.63 |
17.9 |
39.0 |
0.18 |
0.56 |
|
Sunflower Seeds, Oil Type* |
94.9 |
121.0 |
1.42 |
1.03 |
17.9 |
39.0 |
0.18 |
0.56 |
|
Sunflower Silage, Low-oil Variety** |
30.0 |
61.0 |
0.61 |
0.69 |
11.1 |
42.0 |
0.8 |
0.3 |
|
Sunflower Silage, High-oil Variety** |
30.0 |
66.0 |
0.35 |
0.42 |
12.5 |
39.0 |
1.50 |
0.3 |
|
*Adapted from Lardy and Anderson, 2003. |
||||||||
Sunflower Residue
Sunflower residue is useful for aftermath grazing by beef cows. Nutritional value of the head is greater than the stalk. Unharvested sunflower heads containing seeds will be highly sought by beef cows. Supplementation may be required if the volume of residue is limited and nutrient quality decreases rapidly after head material is consumed.
Sunflower Screenings
Sunflower screenings from both confection and oil seed plants are often available at competitive prices. Nutrient content varies widely with the amount of meats, which are high in fat and protein, and hull, which is low in nutrient content and digestibility. Screenings are best used in modest growing or maintenance diets when animal performance is not critical. The presence of sclerotia bodies in the screenings does not appear to be a problem for palatability, nutrient content or animal performance.
Sunflower Hulls
Sunflower hulls are low in protein and energy and should be used only as a low-quality roughage source or as bedding for beef cattle.
Summary
Sunflower meal is a useful protein source for growing and finishing cattle. Similarly, beef cows can be provided supplemental protein effectively with sunflower meal. Sunflower meal may be especially useful in diets where degradable protein is required, such as lower-quality forage or high corn finishing rations. The increased bulk of this relatively high-fiber meal may affect transportation and storage, but ruminants are positioned to be more tolerant of high fiber levels than other species. Other sunflower products can be used effectively in ruminant diets when consideration is provided for nutritional differences.
U.S. Grades and Standards for Sunflower
Definition of Sunflower Seed
Grain that, before the removal of foreign material, consists of 50% or more of cultivated sunflower seed (Helianthus annuus L.) and not more than 10% of other grains for which standards have been established under the U.S. Grain Standards Act.
Definition of Other Terms
Cultivated sunflower seed – Sunflower seed grown for oil content. The term seed in this and other definitions related to sunflower seed refers to both the kernel and hull, which is a fruit or achene.
Damaged sunflower seed – Seed and pieces of sunflower seed that are badly ground-damaged, badly weather-damaged, diseased, frost-damaged, heat-damaged, mold-damaged, sprout-damaged or otherwise materially damaged.
Dehulled seed – Sunflower seed that has the hull completely removed from the sunflower kernel.
Foreign material – All matter other than whole sunflower seeds containing kernels that can be removed from the original sample by use of an approved device and by handpicking a portion of the sample according to procedures prescribed in the USDA’s Federal Grain Inspection Service instructions.
Heat-damaged sunflower seed – Seed and pieces of sunflower seed that are materially discolored and damaged by heat.
Hull (husk) – The ovary wall of the sunflower seeds.
Kernel – The interior contents of the sunflower seed that are surrounded by the hull.
Basis of Determination
Each determination of heat-damaged kernels, damaged kernels, test weight per bushel and dehulled seed is made on the basis of the grain when free from foreign material. Other determinations not specifically provided for in the general provisions are made on the basis of the grain as a whole, except the determination of odor is made on either the basis of the grain as a whole or the grain when free from foreign material.
Table 19 lists the U.S. grade requirements for sunflower according to the Federal Grain and Inspection Service. These requirements became effective Sept. 1, 1984. The table lists the minimum limit for test weight and the maximum for damaged and dehulled seed. U.S. grades for both oilseed and nonoilseed classes of sunflower are determined with the requirements listed below.
Table 19. Grade and grade requirements for sunflower.
|
Grade |
Minimum |
Maximum limits of damaged sunflower seed |
||
|
Heat damage |
Total |
Dehulled seed |
||
|
(pounds) |
- - - - - - - - (percent) - - - - - - - - |
|||
|
U.S. No. 1 |
25.0 |
0.5 |
5.0 |
5.0 |
|
U.S. No. 2 |
25.0 |
1.0 |
10.0 |
5.0 |
|
U.S. Sample grade – U.S. sample grade shall be sunflower seed that: (1) Does not meet the requirements for the grades U.S. Nos. 1 or 2; or (2) In a 600-gram sample, contains eight or more stones that have aggregate weight in excess of 0.20% of the sample weight, two or more pieces of glass, three or more crotalaria seeds (Crotalaria spp.), two or more castor beans (Ricinus Communis), four or more particles of an unknown substance(s), or 10 or more rodent pellets, bird droppings or an equivalent quantity of other animal filth; or (3) Has a musty, sour or commercially objectionable foreign odor; or (4) Is heating or otherwise of distinctly low quality. |
||||
Source: Federal Grain and Inspection Service-USDA
Glossary
Achene — The sunflower fruit consisting of hull and “seed;” a small, dry, one-seeded fruit that does not open at maturity.
Apothecium (pl. apothecia) — Cup- or saucer-shaped fruiting structure of some fungi.
Ascospore — Fungal spore borne in a structure within the apothecium.
Annual — Plant in which the entire life cycle is completed in a single growing season.
Bract — Modified, reduced leaf structure beneath ray flowers on sunflower head.
Canker — Sharply defined dead area of tissue on stem.
Corolla — Collective term for petals of the sunflower.
Cytoplasmic Male Sterility — Male sterility inherited through hereditary units in the cytoplasm, rather than through nuclear inheritance.
Defoliate — To remove leaves of a plant.
Dehull — Removal of outer seed coat (hull) from the “seed.”
Depredation — A plundering or despoiling; robbery.
Desiccant — A dry-down or defoliating chemical.
Disk Flower — Tubular flowers that compose the central part of the sunflower head; produce the seeds.
Fungicide — A chemical or physical agent that kills fungi.
Fungus (pl. fungi) — A group of organisms that lack chlorophyll and that obtain food through absorption, frequently from plants.
Herbicide — A chemical or physical agent that kills plants.
High Oleic — Oilseed sunflower that contains a trait for high oleic fatty acid content in its oil. A premium oil used in the snack food industry.
Host — The organism affected by a parasite or disease.
Hybrid — The offspring of two unlike parents.
Insecticide — A chemical or physical agent that kills insects.
Instar — Any stage of insect development; larval growth stage.
Involucral Bract — An individual bract within a distinct whorl of bracts that subtend the flowering part of a plant.
Kernel — Term used for true seed in processing, preferred to “nutmeat.” The sunflower seed is neither “nut” nor “meat.”
Larva (pl. larvae) — The preadult form of an insect.
Nonoilseed — Preferred term, equivalent to nonoil sunflower or confectionery sunflower.
NuSun — Term that describes the mid-oleic sunflower oil. It is lower in saturated fat (less than 10%) than linoleic sunflower oil and has higher oleic levels (55% to 75%) with the remainder being linoleic (15% to 35%)
Oilseed — Preferred term, equivalent to oil sunflower.
Open Pollinated — Naturally pollinated by selfing or crossing between two related strains.
Perennial — A plant that continues its growth from year to year, not dying after once flowering.
Petiole — The stalk of the leaf.
pH — Expression of acidity or alkalinity of soil or water.
Physiological Maturity — Stage at which a seed has reached its maximum dry weight.
Pollinator — Insect that carries pollen from plant to plant.
Pupa (pl. pupae) — The stage between larva and adult in some insects.
Ray Flower — Flattened, ray shaped flowers on margins of sunflower head. Commonly referred to as the petals. These are sterile and do not produce achenes.
Receptacle — Fleshy, thickened part of sunflower head just above the stem that bears the flower parts.
Sclerotium (pl. sclerotia) — The hard, resting bodies of certain fungi.
“Seed” — True seed in sunflower is the kernel; however, “seed” commonly is used to describe the kernel plus hull, which is equivalent to the achene.
Self Compatability — Production of fruits and normal seeds following self pollination.
Spore — Reproductive structure of fungi.
Sunflower — The preferred term, equivalent to sunflowers.
Sun Oil — The preferred term, equivalent to sunflower oil, sunflower seed oil.
Variety — A subdivision of a species; a distinct group of organisms.
Volunteer Plant — Plant arising from seed dispersed from a previous crop.

Published with support from the National Sunflower Association.
NDSU Extension does not endorse commercial products or companies even though reference may be made to tradenames, trademarks or service names.
For more information on this and other topics, see www.ndsu.edu/extension


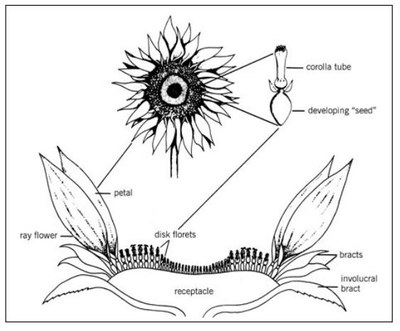





.jpg)

.jpg)


.jpg)


.jpg)


.jpg)



.jpg)


.jpg)




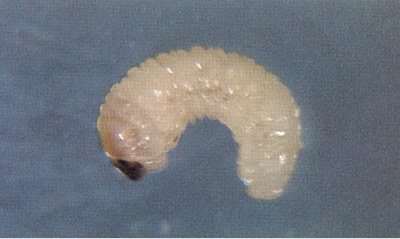



.jpg)

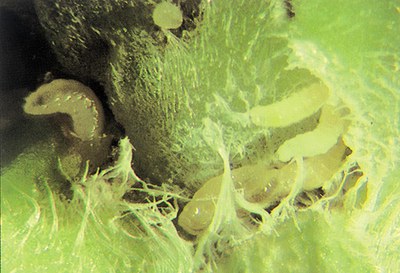
.jpg)

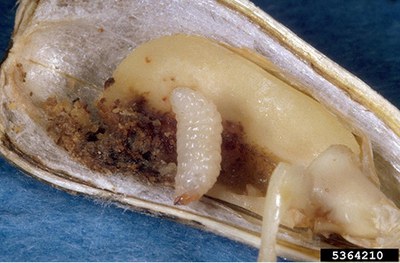
.jpg)




.jpg)









.jpg)









.jpg)

.jpg)

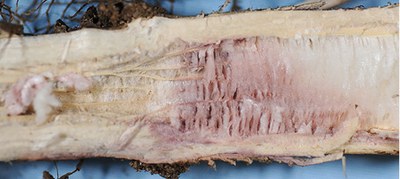
.jpg)



.jpg)







