Late Blight in Potato (PP1849, May 2017)
Availability: Web only
The primary host is potato, but P. infestans also can infect other solanaceous plants, including tomatoes, petunias and hairy nightshade, that can act as source of inoculum to potato.
In North America, late blight survives between seasons in infected seed tubers, cull piles and volunteer plants. Knowing the symptoms and disease cycle of this rapidly destructive disease is necessary to implement management options.
Symptoms
The first symptoms of late blight in the field are small, light to dark green, circular to irregular-shaped water-soaked spots (Figure 1). These lesions usually appear first on the lower leaves. Lesions often begin to develop near the leaf tips or edges, where dew is retained the longest.

Figure 1. Initial symptoms of late blight are small, light to dark green, circular to irregular-shaped water-soaked spots. (Andy Robinson, NDSU/University of Minnesota)
During cool, moist weather, these lesions expand rapidly into large, dark brown or black lesions, often appearing greasy (Figure 2). Leaf lesions also frequently are surrounded by a yellow chlorotic halo (Figure 3).

Figure 2. Late blight lesions expand rapidly into large, dark brown or black lesions, often appearing greasy. (Andy Robinson, NDSU/University of Minnesota)

Figure 3. Leaf lesions frequently are surrounded by a yellow chlorotic halo. (Andy Robinson, NDSU/University of Minnesota)
The lesions are not limited by leaf veins, and as new infections occur and existing infections coalesce, entire leaves can become blighted and killed within just a few days. The lesions also may be present on petioles and stems of the plant (Figure 4).

Figure 4. Late blight lesions may be present on petioles and stems of the plant, especially in new growth where moisture persists. (Andy Robinson, NDSU/University of Minnesota)
During active growth, especially in cool, wet weather, a white mildew-appearing area is visible at the edge of the lesions (Figure 5) or along petioles (Figure 6). This is the area where the late blight pathogen actively is producing spores. As the weather changes to warm and dry, these lesions become dry, stop sporulating and become tan (Figure 7).

Figure 5. During active late blight growth, especially in cool, wet weather, a white mildew-appearing area is visible at the edge of the lesions. (Eugenia Banks, Ontario Ministry of Agriculture and Food)

Figure 6. Actively growing late blight can cause a white mildew-appearing area along petioles. (Neil Gudmestad, NDSU)

Figure 7. Brown, dry lesions can develop following warm and dry weather. (Neil Gudmestad, NDSU)
A pale green to yellow border often surrounds the lesions. Severely infected fields often produce a distinct odor.
Late blight infection of tubers is characterized by irregularly shaped, slightly depressed areas that can vary considerably from brown to purplish of variable size on the skin (Figure 8). These symptoms may be less obvious on russet and red-skinned cultivars.

Figure 8. Late blight infection of tubers is characterized by irregularly shaped, slightly depressed areas that can vary considerably from brown to purplish of variable size on the skin. (Eugenia Banks, Ontario Ministry of Agriculture and Food)
A tan to reddish-brown, dry, granular rot is found under the skin in the discolored areas and extending into the tuber usually less than ½ inch (Figure 9). The extent of the rotting in a tuber depends on the susceptibility of the cultivar, temperature and length of time after the initial infection.

Figure 9. Late blight causes a tan to reddish-brown rot found under the skin. (Jeff Miller, Miller Research LLC).
The margin of the diseased tissue is not always distinct but can be, particularly in seed potatoes that have been stored at cold storage temperatures (Figure 10). However, the margin between late blight-infected tissue and healthy tissue frequently is marked by a brown fingerlike extension into the healthy flesh of the tuber.

Figure 10. The margin of the diseased tissue is not always distinct but can be, particularly in seed potatoes that have been stored at cold storage temperatures. (Francisco Bittara Molina, NDSU)
Infections of other tuber rot organisms such as soft rot bacteria, pink rot or leak frequently may use late blight-affected areas as infection courts and often progress more rapidly than P. infestans, making diagnosis difficult.
Positive identification of late blight can be made by microscopic examination of sporulating samples from infected leaves or tubers. Identification of nonsporulating samples and identification of late blight genotypes can be done by polymerase chain reaction, commonly referred to as PCR. This service is available at NDSU.
Some late blight genotypes have been documented to have resistance to the fungicide mefenoxam/metalaxyl. When late blight is confirmed, determining the late blight genotype is essential to ensure effective chemical treatments. Contact the local Extension Service for current information on fungicide-resistant late blight genotypes.
Disease Cycle
P. infestans, the cause of late blight, is a heterothallic fungal-like pathogen, meaning two mating types are required for sexual reproduction and are referred to as A1 and A2. The pathogen is an obligate parasite that cannot survive without a living host.
However, in some areas of the world where both mating types of the pathogen are present, a sexual spore that is capable of soil survival is produced. Although soil survival is not known to occur definitively in the U.S., anecdotal evidence indicates that sexual combination has occurred, suggesting soil survival is possible.
Infected potato tubers are the primary source of inoculum for P. infestans. That includes potatoes in storage, infected tubers missed during harvest that remain unfrozen during the winter (volunteers), seed tubers and infected cull piles, and P. infestans on other host plants. The pathogen can be transmitted from infected seed tubers to newly emerging potato plants (Figure 11), where it produces airborne spores that can move to neighboring plants.
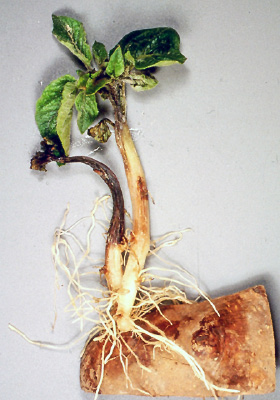
Figure 11. Late blight infected seed often decays, but can move up the seed piece into the stem. (Courtesy D. Inglis; Reprinted from Schumann and D’Arcy, 2000)
Schumann, G. L., and D’Arcy, C. J. 2000. Late blight of potato and tomato. The Plant Health Instructor. American Phytopathological Society. doi: 10.1094/PHI-I-2000-0724-01
The late blight pathogen is favored by free moisture and cool to moderate temperatures. Night temperatures of 50 to 60 F and day temperatures of 60 to 70 F are most favorable for disease development. Free water from rain, dew and overhead irrigation sprinkler irrigation all provide the water necessary for pathogen infection and development.
Spores develop in three to five days and require 12 hours of free moisture for infection to occur. Lesions on leaves and stems become visible as small flecks within a few days after infection.
The lesions expand to water-soaked, gray-green areas on the leaf and sporulate if conditions are favorable. The spores are carried by wind and rain to healthy plants, where the disease cycle begins again. A disease cycle can occur every five to seven days, resulting in rapid spread and movement of late blight.
Tubers are infected by spores washed from lesions to the soil. Spores germinate and swim to tubers in free water and infect primarily the eyes. Tuber infections are characterized by patches of brown to purple discoloration on the potato skin. Cutting just below the skin reveals a dark, reddish-brown, dry, corky rot.
Management
Effective control of this disease requires implementation of an integrated disease management approach. Late blight is a community disease, and effective management requires community management. Here are methods to help control the disease:
- Destroy all cull and volunteer potatoes.
- Plant late blight-free seed tubers.
- Do not mix seed lots because cutting can transmit late blight.
- Use a seed piece fungicide treatment labeled for control of late blight (current list of fungicides can be found in the NDSU “Fungicide Guide,” PP622). Recommended seed treatments include Revus, Reason and mancozeb.
- Avoid planting problem areas that may remain wet for extended periods or may be difficult to spray (the center of the pivot, along powerlines and tree lines).
- Avoid excessive and/or nighttime irrigation.
- Eliminate sources of inoculum such as hairy nightshade weed species and volunteer potatoes.
- Scout fields regularly, especially in low, wet areas, along tree lines, at the center of the pivot and other areas that remain wet for longer periods where late blight first may occur.
- Use foliar fungicides on a regular and continuing schedule. Once late blight is present, only foliar fungicide applications can manage late blight in the field. (A current list of fungicides can be found in the NDSU “Fungicide Guide,” PP622).
- Keep up to date on late blight forecasts. In our region, the NDSU Potato Blightline operates during the growing season and provides weekly late blight updates and forecasting.
- Quickly destroy hot spots of late blight.
- Kill vines completely two to three weeks before harvest.
- Applying phosphorous acid to potatoes after harvest and before piling can prevent infection and the spread of late blight in storage.
- Monitor home garden and market tomatoes near you for late blight. Late blight can move from these local sources to potato fields.


May 2017

