Checklist of Potato Emergence Problems (A2020, May 2021)
At times, potato growers may experience poor emergence of potato plants. Potato plants may not emerge properly for a number of reasons.
This publication is intended to provide a list of common problems that can cause poor potato emergence and stand. Utilizing this list can help growers more rapidly identify the cause and improve management of the crop and subsequent crops.
Planting healthy seed is one of the most important factors that allow growers to produce a high-quality and high-yielding potato crop. Certified seed should give optimum emergence when handled and suberized properly, especially keeping tubers free of bruises or injuries wounds. Optimum soil conditions will increase the probability of a successful plant stand.
Soil temperature and moisture are key factors in optimizing crop emergence. Ideally, the soil temperature and the seed temperature should be the same at planting; ideally 50 to 55 F (10 to 13 C). As soils warm, sprout growth becomes most rapid at 68 to 72 F (20 to 22 C).
Favorable soil moisture should be in the range of 75% to 85% available soil water. Seed treatments are available to reduce the incidence of fungal pathogens such as Fusarium, Helminthosporium and Rhizoctonia, but not for bacterial pathogens.
Slow emergence often results from planting in nonideal conditions or using lower-quality seed. But sometimes a poor stand follows high-quality seed and perfect planting conditions.
For example, planting good seed in a wet soil, either hot or cold, favors bacterial soft rot, which may result in poor stand. Or, planting good seed in ideal conditions, a poor stand can occur because of herbicide carryover in the soil or herbicide residues in the seed.
Potato emergence can be affected a number of ways. The following checklist is of common factors that occur before or after planting that reduce seed emergence.
Seed Rot Caused by Bacterial/Fungal Pathogens
Tuber soft rot (Pectobacterium carotovorum subsp. carotovorum)
This bacterium is found in all agricultural soils and in surface water. Soft rot is favored by free water and high temperatures.
Soft rot bacteria are present in tuber lenticels and penetrate tubers through wounds or bruises. Fusarium infection of seed can predispose seed to bacterial decay. This can happen when seed potatoes are left in a truck on warm days for 24 hours or longer or planted in warm and wet soil.
Rotted tissue is brown, mushy, slimy, water soaked and surrounded by a black margin. Infected seed often has a foul odor and seed rots quickly in the field, leaving gaps in rows.
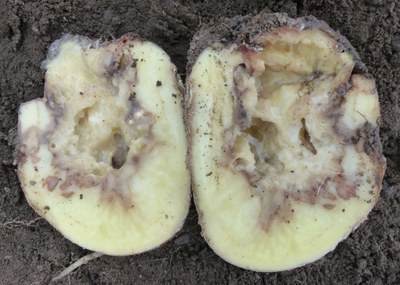
Blackleg (Pectobacterium atrosepticum)
This pathogen is primarily seed borne. Rot starts at the stem end and extends into the tuber. Infected flesh first appears cream-colored but turns darker, grayish black and mushy with time.
Secondary bacteria that invade affected tubers give off a fishy smell. Planting infected tubers may result in seed rot and pre-emergence or postemergence stem rot called blackleg.
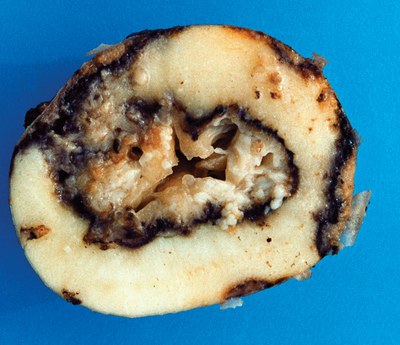
Dickeya (Dickeya dianthicola)
The symptoms of Dickeya tuber rot are very similar to blackleg; however, Dickeya tuber rot often looks “cheesy” and it is usually not as smelly as blackleg. Healthy-looking seed tubers may carry the Dickeya bacterium in a dormant state; such tubers usually rot and plants wilt when spring temperatures are close to 77 F (25 C.)
Fusarium seed piece decay (Fusarium spp.)
Tuber infection occurs at wound sites. External tuber lesions are sunken and shriveled with concentric wrinkles. Rotting flesh may be brown or gray to black, dry and crumbly.
Cracks often form in the lesions and cavities often are lined with white, pink, yellow or blue mold that develops on the rotted tissue. Infected seed pieces may be partially or completely destroyed in the soil, resulting in uneven stands.
Late blight in seed (Phytophthora infestans)
External tuber lesions are slightly sunken brown to purplish areas of variable size. The internal symptom is a tan to brown granular dry rot. No clear separation occurs between infected and healthy tissue.
Often, soft rot bacteria invade infected tubers. Infected seed may rot in the field or weak, sick plants may emerge. Later, lesions that develop on these plants produce thousands of spores that can start an epidemic if the weather is cool and wet.
Eye or Sprout Infections
Black dot (Colletotrichum coccodes)
This fungus produces light brown blemishes with microsclerotia on the tuber skin. Later, unsightly dark brown patches can develop.
Lesions tend to be irregularly shaped and often without a defined outline. The black dots (microsclerotia), which differentiate this disease from silver scurf, are easily seen under a hand lens (10x magnification).
In very susceptible varieties, the lesions are black and cover larger areas of the tuber. Eyes within affected areas produce weak sprouts, resulting in poor stands if severely infected seed is planted.
Rhizoctonia brown cankers on sprouts (Rhizoctonia solani)
A symptom of this disease is the formation of brown cankers on sprouts. Brown cankers may pinch off sprouts, resulting in poor emergence.
Severe mosaic virus
This is most often seen in potato virus Y (PVY)-infected plants that emerge and die prematurely, which results in poor stands. Infected plants exhibit a necrotic leaf drop symptom of lower leaves.
Zebra chip
Tubers infected by C. Liberibacter solanacearum, the cause of zebra chip, do not sprout or sprout with hair sprouts.
Physiological Conditions
Chilling injury
This can occur by tuber exposure to temperatures between 31 and 34 F (minus 0.5 to 1.1 C). This may occur while the crop is still in the ground before harvest, in transit or in storage.
Chilling injury is not visible on the tuber surface but causes pinkish-red to reddish-brown or gray discoloration of the flesh. (The reddish-brown discoloration symptom is called mahogany browning).
The vascular tissue is very sensitive to chilling injury. Chilled cells are not frozen, and tissues do not break down rapidly. Chilled potatoes should not be used for seed.
Cut surfaces do not heal properly, and seed-piece decay is likely. Sprouts from chilled potatoes usually die off.
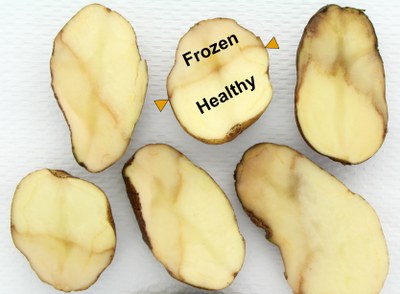
Freezing injury
Soil temperatures below 28 F (minus 2 C) for several hours kill the tuber tissue closer to the soil surface. Symptoms become visible as the tubers begin to thaw, resulting in free moisture (weeping) on the tuber surface.
Later, cellular breakdown causes the tissue to turn brown, gray or black. A distinct line is visible between healthy and frozen tissue.
Frozen sprouts
Freezing temperatures kill emerging sprouts. New sprouts regrow from healthy tissue, emerging at a 90-degree angle to the stem. This delays emergence for at least a week and the stand is not uniform.
Bruising
Pressure bruising in storage or bruising seed potatoes before planting increases seed piece decay by providing entry points for bacteria or pathogens such as bacterial soft rots and Fusarium spp. or may reduce sprouting due to eye damage.
Physiologically old seed
Seed that accumulated many growing degree days or was stressed in the field prior to harvest, was not properly stored or was stressed by excessive handling can produce recognizable symptoms. It has sprouts, or second growth, and/or it can be spongy or dehydrated.
Planting seed with these symptoms can result in missing plants, slow emergence, multiple stems and a nonuniform stand in the field. In the field, hot soil temperature is the main cause of seed aging.
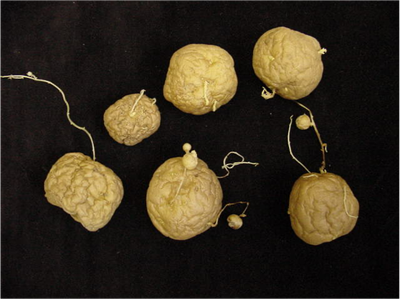
- Little potato disorder or no top: New tubers are produced directly from the sprouts, without emergence. (Figure 13a)
- Hair sprouts: High soil temperature toward the end of the growing season causes this disorder. Hair sprout disorder may develop on physiologically old tubers along with the formation of little potatoes resulting in poor stands. Hair sprouting is also a symptom of infection with the black dot fungus. (Figure 13b).
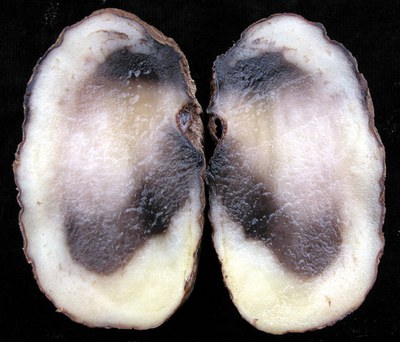
Blackheart
This can occur during tuber development and/or storage by the lack of oxygen, leading to dark, necrotic cavities. Increased temperature can intensify blackheart, especially after vine kill and when soils are saturated. Seed with severe blackheart disorder often rots before emergence.
Sprout inhibitor contamination
- Chlorpropham (CIPC) contamination in storage: The sprouts of seed contaminated by residues of CIPC in storages are cauliflower-shaped and will not produce plants.
- Maleic Hydrazide (MH-30) residues in seed from contact during previous growing season: MH-30 suppresses sprouting, and suppression of emergence depends on the residue level and size of the tubers. The larger the tubers, the higher the concentration of residues. Residues decline with time and sprout development occurs, but growth is substantially delayed.
- If accumulated in sufficient quantities, sprout-inhibiting compounds may cause delayed emergence of seed tubers.
Herbicide issues
Herbicide carryover in soil: Patterns of soil carryover will have consistent injury symptoms in the areas of the field or where the herbicide carryover is a problem. Issues can be in the entire field or specific areas with higher herbicide carryover based on soil characteristics or previous herbicide application.

- Disrupted plant growth caused by herbicide carryover in soil. (Andy Robinson, NDSU/University of Minnesota)
Herbicide residue in seed: Seed may not sprout or have cauliflowerlike sprouts and/or candelabralike stems caused by herbicides, causing a loss of apical dominance. That is most commonly caused by glyphosate.
Emergence most often will appear as scattered injury throughout the seed lot. Some plants will look normal; others will show varying levels of herbicide injury symptoms.
Herbicide damage by soil type: Soils with low organic matter and high pH can cause herbicides to be more active and increase injury symptoms. Some varieties are more susceptible to herbicides such as metribuzin.
Chloroacetamide herbicide injury: Cool and wet conditions will increase plant uptake and prolong plant metabolism, causing slow growth and malformed leaves. The active ingredients metolachlor and dimethenamid can cause slowed emergence.
Variety Characteristics
Apical dominance
This refers to tubers with eyes concentrated at the bud end (e.g. ‘Ambra’) or that have a low number of eyes (e.g. ‘Bannock Russet’). This results in blind seed pieces when the seed is cut. Blind seed pieces will not sprout, resulting in poor emergence.
Low dry matter
Varieties with low dry matter such as ‘Colomba’ or ‘Red Norland’ are prone to tuber rot if the seed is treated with a liquid seed treatment. The cut surface stays wet and does not suberize but rots. Tubers that are cut wet are more difficult to suberize and more prone to rot.
Late, erratic dormancy
The varieties ‘Waneta’ and ‘Bannock Russet’ are prone to these problems. Emergence and stands are often nonuniform due to differential sprouting of bud-end and lateral eyes.
Soil/seed conditions
Extremely high soil temperature can burn the sprouts. This often is seen in sandy, hot soil when potatoes are emerging. The sprout number increases and new sprouts re-grow, but emergence is delayed and not uniform. Leaves can become malformed and appear to have herbicidelike injury.
Anaerobic conditions due to field flooding induce seed rotting usually caused by soft rot (see tuber soft rot).
Planting warm, freshly cut seed into cold soils can lead to poor stands, as can planting cut seed into cold, wet soils. The temperature difference between soil and seed pulp should be no more than 10 F (5 to 6 C).
Planting wet, pre-cut seed kept in storage with poor ventilation and high humidity can result in poor stands.
Seed cutters not calibrated properly can increase wedge and sliver pieces that are blind. Seed cutters not properly sharpened do not cut cleanly, making suberization difficult.
Dried vs. suberized cut seed: Cut seed that is not properly suberized but kept too dry can have dried skin (not suberized) and be prone to cracking and allowing disease entry and rot.
Nematodes
Nematode injury to emerging stems
Root lesion nematodes (Pratylenchus penetrans) at high levels can cause significant injury to roots and allow secondary pathogens to infect, slowing emergence. This typically occurs when potato seed is planted early and has a prolonged emergence period.

- Root lesion nematode injury can cause varied emergence. (Andy Robinson, NDSU/University of Minnesota)

- Root lesion nematode injury can cause poor emergence and may be confused with herbicide carryover in soil. (Andy Robinson, NDSU/University of Minnesota)
Planting
Planter issues that are row specific
Many things can happen at planting that effect seed emergence and stand that are related to the planter setup. Some common issues that happen are fertilizer misses, guess rows not properly lined up, planter pattern not lined up, variable seed depth, planting too fast (can cause skips or doubles), broken or malfunctioning planter cups and running out of seed.
Summary
A number of issues can cause poor emergence or reduced stand count. This list briefly explains and demonstrates some common issues.
Some problems may be confused with other problems. For diseases and herbicides, laboratory testing can confirm the presence of one of these issues. Immediately getting good samples to a laboratory will improve the chances of proper identification.
At times, problems that cause a poor emergence cannot be identified. In these cases, contact your local Extension specialist or potato expert to help with diagnosis.




NDSU Extension does not endorse commercial products or companies even though reference may be made to tradenames, trademarks or service names.