Canola Diseases: Clubroot (PP1998, Dec. 2020)
Clubroot is a serious disease threat to canola production in the U.S. and Canada. In 2013, the disease first was identified in Cavalier County, located in the upper northeastern part of North Dakota. Since 2013, other counties are being monitored for detection of clubroot in North Dakota.
Once established, no economical ways to eliminate the pathogen from the soil exist. Early detection of the disease and use of available management tools are critical to slow the spread and limit yield loss from clubroot.
Cause
The pathogen causing clubroot, Plasmodiophora brassicae, is a soil-borne organism in the taxonomic kingdom Protista. Compared with other pathogen groups (such as fungi, bacteria and viruses), Protista pathogens are relatively rare, and their biology and the management strategies to manage them are somewhat unique.
P. brassicae affects cruciferous plants including crops such as broccoli, Brussels sprouts, cabbage, camelina, canola, cauliflower, mustards, radishes and kale, and weeds such as shepherd’s purse, pepperweed, Western wallflower, tumble mustard, stinkweed and volunteer canola. The pathogen is genetically variable and can develop different pathotypes (similar to races or strains) that overcome resistance genes. Consequently, robust weed control and hybrid selection are very important for management.
Signs and Symptoms
Symptoms of clubroot can include wilting, stunting, reduced seed production, a thin stand and premature plant death (Figures 1-3). However, above-ground symptoms do not always occur and are not diagnostic of the disease. Additionally, above-ground symptoms of clubroot resemble many other ailments, including moisture stress (flooding or drought) and infection with other diseases (blackleg, sclerotinia stem rot, etc.).

- Figure 1. Area of field with clubroot resulting in a stunting, wilting and thin stand. (Venkata Chapara, NDSU)
P. brassicae colonizes root systems, compromising the moisture and nutrient uptake of the plant. When scouting, whole root masses should be dug up and carefully examined, rather than pulled at the base of the stem. Infected roots will appear deformed with swollen areas of tissue called galls. Galls will be small as they begin to develop but may be up to 1 inch in diameter by the time of maturity. Infected roots may have just one or a few galls in discrete areas of the root tissue (resembling clubs) or numerous galls that impact nearly the entire root system (Figures 4 and 5). Galls commonly lose integrity as the season progresses, and by plant maturity are brittle and dusty when broken.
Several weeks after swathing, galls may have decayed and no longer be visible. The remaining roots appear dark brown rather than have a normal and healthy whitish appearance. Severely infected plants/stems will have very little root tissue remaining and can be hand-pulled from the soil easily.
Disease Cycle
The pathogen survives as robust resting spores, which can remain viable for more than a decade in the soil. In the spring, resting spores germinate to produce motile zoospores that swim through water in the soil.
Zoospores encyst on root hairs of susceptible plants and form a cell that (under a microscope) resembles an amoeba, called a plasmodium. The plasmodium invades cells and divides to become more zoospores that re-infect additional root tissue or are released into the soil and can infect neighboring plant roots.
Once infection occurs in the cortical tissue (central cell layer), the pathogen produces secondary plasmodia that cause plant cells to divide and swell, resulting in gall formation on the roots. The plasmodia eventually divide into millions of resting spores that are released back into the soil when galls rapidly disintegrate and are decayed by soil microbes.
High soil moisture, acidic soil (low pH) and temperatures around 68 to 77 F favor infection and disease severity. Poorly drained areas or parts of fields commonly flooded are more prone to infection. Although infection (and yield loss) can occur in soils at any pH, those with pH above 7.2 may have less damage.
P. brassicae is most commonly spread during its resting spore stage through infested soil that is stuck on farm equipment or other vehicles that enter infested fields. However, strong wind gusts and flooding also have been identified as capable of transferring spores. Consequently, new clubroot infestations are commonly observed first in field entrances (from soil moved on equipment), in low-lying or flood-prone areas and/or along shelter belts.
Management
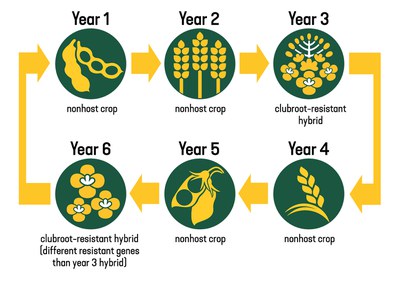
The best approach to managing clubroot is a combination of rotation of genetics for resistance, longer crop rotations, sanitation and excellent weed control. We recommend a six-year plan with two years of nonhost crops followed by a clubroot-resistant canola hybrid, then another two years of nonhost crops followed by a clubroot-susceptible canola hybrid or a clubroot-resistant hybrid that contains different resistant genes than the hybrid planted earlier (Figure 6).
Minimize Spread
Limiting movement of soil will help prevent the spread of the pathogen within a field known to have clubroot and also prevent introduction of the pathogen into new fields. Reducing soil disturbance and movement (such as by limiting tillage, limiting trips across the field, etc.) minimizes the dispersal of resting spores.
Working an infested field last reduces opportunity to spread large amounts of infested soil to other fields on the farm. Sanitation of equipment before moving equipment from an area known to be infested by clubroot is important to limit the spread of the pathogen into other canola-growing areas in the region. For more information on sanitation, visit the Canola Council of Canada’s publication “Managing Clubroot: Equipment Sanitation Guide” at www.canolacouncil.org/download/130/agronomy-guides/2555/clubroot_sanitation_guide-2.pdf.
Crop Rotation
Although resting spores can survive for more than a decade, crop rotation is still beneficial. The Canola Council of Canada recommends a minimum of a two-year break between canola crops, and a three-year break with nonhost crops can reduce inoculum levels. Lengthening crop rotations also will slow the development of new pathotypes that can overcome genetic resistance.
Manage Weeds and Volunteer Canola
The clubroot pathogen can reproduce on volunteer canola and cruciferous weedy species, including all mustards, shepherd’s purse and stinkweed. Effective weed control maximizes the benefits of crop rotation.
Genetic Resistance
Clubroot-resistant hybrids will help limit disease development, severity and yield loss. Resistant hybrids also have the added benefit of limiting the buildup of resting spores in the soil through time.
However, the pathogen causing clubroot is highly variable, and the same resistant hybrid should not be used in consecutive canola crops. This is particularly important in fields with severe infestations. Rotation of resistance genes and hybrids is critical for protecting the effectiveness of the resistance genes and limiting yield loss.
Fungicides
Foliar fungicides and seed treatments have limited effect on clubroot, but soil drench applications of several fungicides provide some management of the disease. Always obtain the most current information if considering a fungicide.
Soil Amendments
Lime has been used to increase the soil pH above 7, making the soil less favorable for pathogen development. However, the amount of lime required to increase the pH to levels that would reduce clubroot severity make this an impractical option for commercial use in canola.
Soil Fumigation
Although this has been done in high-value cruciferous crops, fumigation is not practical for canola production. Fumigation is very costly and destroys the natural soil microbial community by indiscriminately killing soil microbes, and many fumigants are banned due to environmental concerns.
Trap Cropping
Trap cropping, the practice of planting a susceptible crop that attracts infection from a pathogen and then killing the crop before the pathogen can develop a secondary or survival structure, has been used for disease management in some cropping systems. This possibly could have some effect, (for example, using summer rape) but it is costly and dependent on a growing season long enough to plant and destroy a crop prior to planting canola.
Additionally, trap cropping has the risk of potentially increasing the inoculum level of the pathogen if the trap crop cannot be destroyed at the appropriate time. At this time, the practice is not thought to be practical in our environment and production system.
Sampling Your Fields
To obtain a soil sample from your field, collect one scoop or soil core (using a soil core sampler) from the upper 2- to 6-inch layer at five places. These samples should be collected following a “W” pattern starting at the main entrance to the field (approach) and be spaced approximately 300 feet apart.
In addition to the main entrance to the field, other areas to be sampled should include low spots and areas where canola plants are dying prematurely. Combine the scoops or soil cores and air-dry the sample indoors overnight. The sample can be mailed to the NDSU Plant Diagnostic Laboratory for further processing.
Published with the support of Northern Canola Growers Association.