| Oakes Irrigation Research Site |
| Carrington Research Extension Center
* North Dakota State University |
| P.O. Box 531, Oakes, ND 58474-0531, Phone:
(701) 742-2744, FAX: (701) 742-2700, E-mail: |
Blaine.Schatz@ndsu.edu |
|
|
|
|
|
|
Kelly.c.Cooper@ndsu.edu |
|
Leonard.Besemann@ndsu.edu |
|
|
|
|
|
|
| Impact of Inoculation Timing on Seed Yield
and Quality in Confection Sunflowers |
| M. Wunsch, B.
Kraft, M. Schaefer and S. Kallis, L. Besemann and H. Eslinger |
|
|
| Methods |
|
|
|
|
|
|
|
|
|
|
|
|
|
| General Agronomics: The study was on a Maddock sandy loam soil
type. The soil fertility results from the fall soil test: pH = 7.5; 1.6%
organic matter; soil N 22 lbs/acre; soil P = 25 ppm, soil K = 181 ppm, soil S
24 lb/acre and soil Zn = 1.09 ppm. The previous crop was spring wheat and the
tillage operation consisted of disking once followed by two passes with a
multiweeder to smooth the seedbed and incorporate the herbicide Trust (4
lbs/gal trifluralin; Winfield Solutions) applied at 1 pt/acre preplant
incorporated June 1. |
|
|
| Experimental design: A
completely randomized block design with nineteen replicates. The seeded plot
size was 10 feet (center to center) by 20 feet long. The harvested plot size
was ten feet (center to center) by approximately seventeen feet long. There were
four rows per plot and the row spacing was 30 inches. Guard plots (10 feet
wide) were established along all perimeters of the trial. |
|
|
| Planting details: Nuseed Jaguar sunflowers were planted on
June 1 using a Monosem vacuum precision planter. The seeding rate was 3.83
seeds/linear foot of row = 60,000 seeds/acre. The final plant population was
1 plant every 12 inches of row = 17,400 plants/acre. The final population was
established by manual thinning the sunflowers at the V2 growth stage June 17
and June 18. |
|
|
| Disease establishment: Spore solutions were prepared by adding
laboratory-grown ascospores of Sclerotinia sclerotiorum to non-chlorinated
water and adding one to two drops of Tween 20. Hand-held spray bottles were
calibrated to determine how much liquid was released through each squirt of
the bottle, and the spore solution was adjusted so that approximately 30,000
spores were delivered through 3 squirts of the spray bottle. |
|
|
| Inoculation methods R5.1 to R5.3 inoculation timing:
Inoculations were conducted over multiple days such that every head was
inoculated at R5.1 to R5.3 (10 to 30% of the disk flowers blooming or already
bloomed). In each inoculation,
approximately 30,000 spores were delivered through three squirts of the spray
bottle. |
|
|
| Inoculation methods R5.4 to R5.6 inoculation timing:
Inoculations were conducted over multiple days such that every head was
inoculated at R5.4 to R5.6 (40 to 60% of the disk flowers blooming or already
bloomed). In each inoculation,
approximately 30,000 spores were delivered through three squirts of the spray
bottle. |
|
|
| Inoculation methods R5.7 to R5.9 inoculation timing:
Inoculations were conducted over multiple days such that every head was
inoculated at R5.7 to R5.9 (70 to 90% of the disk flowers blooming or already
bloomed). In each inoculation, approximately 30,000 spores were delivered
through three squirts of the spray bottle. Supplemental overhead irrigation
was applied to this trial through a micro-sprinkler misting system, with the
frequency and intensity of irrigation adjusted relative to weather conditions. |
|
|
| Disease assessments: Disease assessments were conducted on
August 25 and August 26 at the R7 growth stage and on September 10 and
September 11 at the R8 growth stage. Each plant was evaluated for the percent
of the head exhibiting Sclerotina head rot. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Harvest and seed yield and quality assessment: Plants were
manually clipped and bagged on September 16 and September 17 as the
sunflowers approached maturity (sunflowers reached R9 on September 11) and
subsequently run through a combine. To adjust yield data to standard moisture
levels, seed moisture levels were assessed from all plots at harvest. The
entire plot was harvested, and plot-level yields were adjusted to a standard
10% moisture level. The percent sclerotia (by weight) in the harvested grain was
assessed by manually removing all sclerotia from a 120-gram subsample of
grain from each plot. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Statistical analysis:
Data were evaluated with analysis of variance except those data sets for
which multiple plots included missing data (Sclerotinia heat rot severity,
shattering incidence and severity; for these data sets, any plots in which no
disease was observed were recorded as missing data). Assumptions of ANOVA:
(1) The assumption of constant variance was assessed with Levene's test for
homogeneity of variances and visually confirmed by plotting residuals against
predicted values. (2) The assumption of normality was assessed with the
Shapiro-Wilk test and visually confirmed with a normal probability plot. (3)
The assumption of additivity of main-factor effects across replicates (no
replicate-by-treatment interaction) was evaluated with Tukey's test for
nonadditivity.The R9 Sclerotinia head rot incidence and severity index data
and % seed over 25/64 sieve for hybrid 12GCF05 exhibited a moderate
deviation from normality due to outliers; a systematic transformation could
not be found that addressed the problem, and the untransformed data were
analyzed. For data that violated model assumptions, a systematic natural-log
transformation [LN(x+1) for data sets with values less than 1, otherwise
LN(x)] was applied. Assessment of whether results
differed by inoculation timing and/or hybrid and whether there was an
interaction between inoculation timing and hybrid:
Analyses were conducted with replicate, main factor, main-factor by replicate
interaction, sub-factor, and sub-factor by main-factor interaction in the
model, with F-tests for replicate and the main factor (hybrid) utilizing
replicate-by-hybrid interaction for the error term. Assessment of
inoculation timing: The impact of inoculation
timing treatments was evaluated separately for each hybrid; where no
significant (P < 0.05) hybrid x inoculation treatment interaction
occurred, it was also evaluated in the combined data across hybrids, with
data from each hybrid considered a
separate replicate (for a total of 16 replicates). Analyses were conducted
with replicate and treatment as main factor effects. Single-degree-of-freedom
contrasts were performed for all pairwise comparisons of isolates; to control
the Type I error rate at the level of the experiment. Analyses were
implemented in PROC UNIVARIATE and PROC GLM of SAS (version 9.3; SAS
Institute, Cary, NC). |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Conclusions: |
|
|
|
|
|
|
|
|
|
|
|
|
|
| * Disease was most
severe when sunflower heads were inoculated when 10-30% and 70-90% of disk
flowers were blooming or had already completed bloom. The reduced disease
development observed when inoculations were conducted when 40-60% of disk
flowers were blooming or already completed bloom may have been caused by
environmental conditions less favorable for disease when sunflowers were
predominantly at this growth stage. |
| * Yields were reduced
0.44 to 0.53% for every 1% increase in the average percent of heads
exhibiting head rot. The impact of disease yield was similar irrespective of
whether inoculations were conducted at R5.1-5.3, R5.4‑5.6, or R5.7-5.9. |
| * The results suggest
that, to be effective, fungicides will probably need to be applied at bloom
initiation when conditions favorable to disease occur. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| This material is based upon work supported by the U.S.
Department of Agriculture, Agricultural Research Service, under agreement No.
58-5442-4-018. Any opinions, findings, conclusions, or recommendations
expressed in this publication are those of the author(s) and do not
necessarily reflect the view of the U.S. Department of Agriculture. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Table 1. Disease, Seed Yield and Seed Quality
Response to Inoculation Timing. |
|
Sclerotinia head rot |
|
|
|
|
|
|
|
Aug. 25-26 | R7 growth stage |
|
Sept. 10-11 | R8 growth stage |
|
|
Test |
Sclerotia in harvested grain |
| |
Incidence |
Severity |
Sev Index |
|
Incidence |
Severity |
Sev Index |
Yield |
weight |
|
---------------------------------%--------------------------------- |
lbs/ac |
lbs/bu |
% by weight |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Non-inoculated |
14 |
a* |
38 |
ab* |
6 |
a* |
|
74 |
a* |
68 |
a* |
51 |
a* |
2715 |
a* |
41.3 |
a* |
3 |
a* |
| Inoculated once at R5.1 to
R5.3 (first third of bloom) |
38 |
b |
38 |
ab |
15 |
b |
|
90 |
b |
85 |
bc |
78 |
bc |
2264 |
b |
41.0 |
ab |
7 |
b |
| Inoculated
once at R5.4 to R5.6 (second third of bloom) |
26 |
a |
33 |
a |
9 |
a |
|
89 |
b |
82 |
b |
74 |
b |
2534 |
a |
41.9 |
ab |
6 |
b |
| Inoculated once at R5.7 to
R5.9 (last third of bloom) |
43 |
b |
41 |
b |
18 |
b |
|
94 |
b |
88 |
c |
83 |
c |
2162 |
b |
40.3 |
b |
9 |
c |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| F: |
14.83 |
4.11 |
14.17 |
|
13.65 |
13.00 |
16.88 |
10.79 |
3.37 |
12.32 |
| P>F: |
< 0.0001 |
0.0127 |
< 0.0001 |
|
< 0.0001 |
< 0.0001 |
< 0.0001 |
< 0.0001 |
0.0282 |
< 0.0001 |
| CV: |
27.5 |
20.6 |
34.4 |
|
6.2 |
6.8 |
10.9 |
10.1 |
4.5 |
34.3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
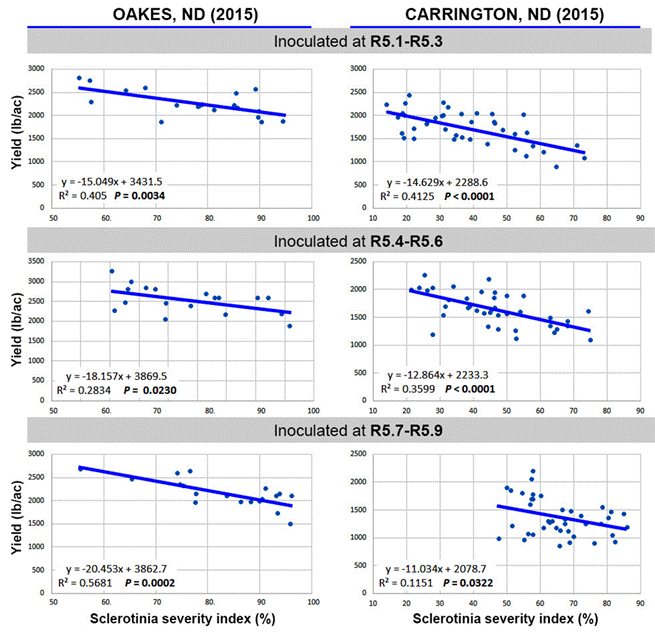
| SCLEROTINIA HEAD
ROT YIELD LOSS RELATIONSHIP RELATIVE TO INOCULATION TIMING |
|
|
|
|
|
| Oakes: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| For every 1% increase in Sclerotinia severity index, yields were
reduced 0.44% when sunflowers were inoculated at R5.1-5.3, 0.46% when
sunflowers were inoculated at R5.4-5.6, and 0.53% when sunflowers were
inoculated at R5.7-5.9. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Carrington: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| For every 1% increase in Sclerotinia severity index, yields were
reduced 0.64% when sunflowers were inoculated at R5.1-5.3, 0.58% when
sunflowers were inoculated at R5.4-5.6, and 0.53% when sunflowers were
inoculated at R5.7-5.9. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Oakes Irrigation
Research Site |
|
| Variety
trials |
Crop
index |
|
Home
page |
|
Report 2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|

